8-Aminoadenosine inhibits Akt/mTOR and Erk signaling in mantle cell lymphoma
- PMID: 20844237
- PMCID: PMC3031409
- DOI: 10.1182/blood-2010-05-285866
8-Aminoadenosine inhibits Akt/mTOR and Erk signaling in mantle cell lymphoma
Abstract
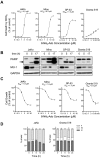
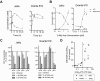
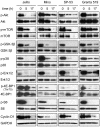
8-Aminoadenosine (8-NH(2)-Ado), a ribosyl nucleoside analog, in preclinical models of multiple myeloma inhibits phosphorylation of proteins in multiple growth and survival pathways, including Akt. Given that Akt controls the activity of mammalian target of rapamycin (mTOR), we hypothesized that 8-NH(2)-Ado would be active in mantle cell lymphoma (MCL), a hematological malignancy clinically responsive to mTOR inhibitors. In the current study, the preclinical efficacy of 8-NH(2)-Ado and its resulting effects on Akt/mTOR and extracellular-signal-regulated kinase signaling were evaluated using 4 MCL cell lines, primary MCL cells, and normal lymphocytes from healthy donors. For all MCL cell lines, 8-NH(2)-Ado inhibited growth and promoted cell death as shown by reduction of thymidine incorporation, loss of mitochondrial membrane potential, and poly (adenosine diphosphate-ribose) polymerase cleavage. The efficacy of 8-NH(2)-Ado was highly associated with intracellular accumulation of 8-NH(2)-adenosine triphosphate (ATP) and loss of endogenous ATP. Formation of 8-NH(2)-ATP was also associated with inhibition of transcription and translation accompanied by loss of phosphorylated (p-)Akt, p-mTOR, p-Erk1/2, p-phosphoprotein (p)38, p-S6, and p-4E-binding protein 1. While normal lymphocytes accumulated 8-NH(2)-ATP but maintained their viability with 8-NH(2)-Ado treatment, primary lymphoma cells accumulated higher concentrations of 8-NH(2)-ATP, had increased loss of ATP, and underwent apoptosis. We conclude that 8-NH(2)-Ado is efficacious in preclinical models of MCL and inhibits signaling of Akt/mTOR and Erk pathways.
Figures
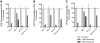

References
-
- Medeiros LJ, Van Krieken JH, Jaffe ES, Raffeld M. Association of Bcl-1 rearrangements with lymphocytic lymphoma of intermediate differentiation. Blood. 1990;76(10):2086–2090. - PubMed
-
- Motokura T, Bloom T, Kim HG, et al. A novel cyclin encoded by a Bcl1-linked candidate oncogene. Nature. 1991;350(6318):512–515. - PubMed
-
- Stilgenbauer S, Winkler D, Ott G, et al. Molecular characterization of 11q deletions points to a pathogenic role of the ATM gene in mantle cell lymphoma. Blood. 1999;94(9):3262–3264. - PubMed
-
- Rizzatti EG, Falcao RP, Panepucci RA, et al. Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the PI3K-AKT, WNT and TGFbeta; signalling pathways. Br J Haematol. 2005;130(4):516–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

