Syntaxin 3 is necessary for cAMP- and cGMP-regulated exocytosis of CFTR: implications for enterotoxigenic diarrhea
- PMID: 20844248
- PMCID: PMC3006332
- DOI: 10.1152/ajpcell.00029.2010
Syntaxin 3 is necessary for cAMP- and cGMP-regulated exocytosis of CFTR: implications for enterotoxigenic diarrhea
Abstract
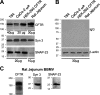
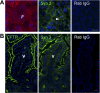
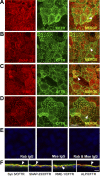
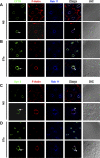
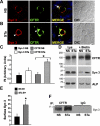
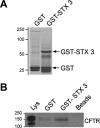
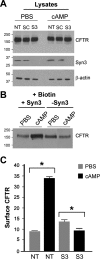
Enterotoxins elaborated by Vibrio cholerae and Escherichia coli cannot elicit fluid secretion in the absence of functional cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels. After enterotoxin exposure, CFTR channels are rapidly recruited from endosomes and undergo exocytic insertion into the apical plasma membrane of enterocytes to increase the number of channels on the cell surface by at least fourfold. However, the molecular machinery that orchestrates exocytic insertion of CFTR into the plasma membrane is largely unknown. The present study used immunofluorescence, immunoblotting, surface biotinylation, glutathione S-transferase (GST) pulldown assays, and immunoprecipitation to identify components of the exocytic soluble N-ethylmaleimide (NEM)-sensitive factor attachment receptor (SNARE) vesicle fusion machinery in cyclic nucleotide-activated exocytosis of CFTR in rat jejunum and polarized intestinal Caco-2(BB)e cells. Syntaxin 3, an intestine-specific SNARE, colocalized with CFTR on the apical domain of enterocytes in rat jejunum and polarized Caco-2(BB)e cells. Coimmunoprecipitation and GST binding studies confirmed that syntaxin 3 interacts with CFTR in vivo. Moreover, heat-stable enterotoxin (STa) activated exocytosis of both CFTR and syntaxin 3 to the surface of rat jejunum. Silencing of syntaxin 3 by short hairpin RNA (shRNA) interference abrogated cyclic nucleotide-stimulated exocytosis of CFTR in cells. These observations reveal a new and important role for syntaxin 3 in the pathophysiology of enterotoxin-elicited diarrhea.
Figures


Similar articles
-
STa and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G.Am J Physiol Cell Physiol. 2005 Sep;289(3):C708-16. doi: 10.1152/ajpcell.00544.2004. Epub 2005 May 4. Am J Physiol Cell Physiol. 2005. PMID: 15872007
-
Syntaxin 8 impairs trafficking of cystic fibrosis transmembrane conductance regulator (CFTR) and inhibits its channel activity.J Cell Sci. 2004 Apr 15;117(Pt 10):1923-35. doi: 10.1242/jcs.01070. Epub 2004 Mar 23. J Cell Sci. 2004. PMID: 15039462
-
cAMP-dependent exocytosis and vesicle traffic regulate CFTR and fluid transport in rat jejunum in vivo.Am J Physiol Cell Physiol. 2003 Feb;284(2):C429-38. doi: 10.1152/ajpcell.00261.2002. Am J Physiol Cell Physiol. 2003. PMID: 12529251
-
Drug-induced secretory diarrhea: A role for CFTR.Pharmacol Res. 2015 Dec;102:107-112. doi: 10.1016/j.phrs.2015.08.024. Epub 2015 Sep 30. Pharmacol Res. 2015. PMID: 26429773 Free PMC article. Review.
-
The cystic fibrosis transmembrane conductance regulator's expanding SNARE interactome.Traffic. 2011 Apr;12(4):364-71. doi: 10.1111/j.1600-0854.2011.01161.x. Epub 2011 Feb 8. Traffic. 2011. PMID: 21223469 Review.
Cited by
-
Molecular motors and apical CFTR traffic in epithelia.Int J Mol Sci. 2013 May 3;14(5):9628-42. doi: 10.3390/ijms14059628. Int J Mol Sci. 2013. PMID: 23644890 Free PMC article. Review.
-
Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine.Am J Physiol Gastrointest Liver Physiol. 2011 Jan;300(1):G82-98. doi: 10.1152/ajpgi.00245.2010. Epub 2010 Oct 28. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21030607 Free PMC article.
-
Linaclotide activates guanylate cyclase-C/cGMP/protein kinase-II-dependent trafficking of CFTR in the intestine.Physiol Rep. 2017 Jun;5(11):e13299. doi: 10.14814/phy2.13299. Physiol Rep. 2017. PMID: 28592587 Free PMC article.
-
Revisiting CFTR Interactions: Old Partners and New Players.Int J Mol Sci. 2021 Dec 7;22(24):13196. doi: 10.3390/ijms222413196. Int J Mol Sci. 2021. PMID: 34947992 Free PMC article. Review.
-
Dihydromyricetin alleviates ETEC K88-induced intestinal inflammatory injury by inhibiting quorum sensing-related virulence factors.BMC Microbiol. 2025 Apr 9;25(1):201. doi: 10.1186/s12866-025-03879-8. BMC Microbiol. 2025. PMID: 40205366 Free PMC article.
References
-
- Ameen N, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol 114: 69– 75, 2000 - PubMed
-
- Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998– 1006, 2007 - PubMed
-
- Ameen NA, Marino C, Salas PJ. cAMP-dependent exocytosis and vesicle traffic regulate CFTR and fluid transport in rat jejunum in vivo. Am J Physiol Cell Physiol 284: C429– C438, 2003 - PubMed
-
- Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg J. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci 112: 887– 894, 1999 - PubMed
-
- Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219– 228, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

