Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal
- PMID: 20844536
- PMCID: PMC2941915
- DOI: 10.1038/nature09347
Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal
Abstract
Specialized cellular microenvironments, or 'niches', modulate stem cell properties, including cell number, self-renewal and fate decisions. In the adult brain, niches that maintain a source of neural stem cells (NSCs) and neural progenitor cells (NPCs) are the subventricular zone (SVZ) of the lateral ventricle and the dentate gyrus of the hippocampus. The size of the NSC population of the SVZ at any time is the result of several ongoing processes, including self-renewal, cell differentiation, and cell death. Maintaining the balance between NSCs and NPCs in the SVZ niche is critical to supply the brain with specific neural populations, both under normal conditions or after injury. A fundamental question relevant to both normal development and to cell-based repair strategies in the central nervous system is how the balance of different NSC and NPC populations is maintained in the niche. EGFR (epidermal growth factor receptor) and Notch signalling pathways have fundamental roles during development of multicellular organisms. In Drosophila and in Caenorhabditis elegans these pathways may have either cooperative or antagonistic functions. In the SVZ, Notch regulates NSC identity and self-renewal, whereas EGFR specifically affects NPC proliferation and migration. This suggests that interplay of these two pathways may maintain the balance between NSC and NPC numbers. Here we show that functional cell-cell interaction between NPCs and NSCs through EGFR and Notch signalling has a crucial role in maintaining the balance between these cell populations in the SVZ. Enhanced EGFR signalling in vivo results in the expansion of the NPC pool, and reduces NSC number and self-renewal. This occurs through a non-cell-autonomous mechanism involving EGFR-mediated regulation of Notch signalling. Our findings define a novel interaction between EGFR and Notch pathways in the adult SVZ, and thus provide a mechanism for NSC and NPC pool maintenance.
Figures
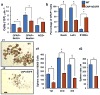
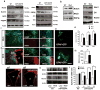
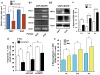
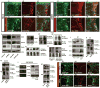
Comment in
-
Stem cells: a balancing act.Nat Rev Mol Cell Biol. 2010 Nov;11(11):753. doi: 10.1038/nrm2990. Epub 2010 Sep 29. Nat Rev Mol Cell Biol. 2010. PMID: 20877398 No abstract available.
-
Neurogenesis: Pathways for population balance.Nat Rev Neurosci. 2010 Nov;11(11):731. doi: 10.1038/nrn2933. Nat Rev Neurosci. 2010. PMID: 20979320
References
-
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. - PubMed
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. - PubMed
-
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. - PubMed
-
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS045702/NS/NINDS NIH HHS/United States
- R01DC006881/DC/NIDCD NIH HHS/United States
- P30 HD040677/HD/NICHD NIH HHS/United States
- R01NS056427/NS/NINDS NIH HHS/United States
- R01 DC006881/DC/NIDCD NIH HHS/United States
- R01 NS045702/NS/NINDS NIH HHS/United States
- P30HD40677/HD/NICHD NIH HHS/United States
- R00 NS057944/NS/NINDS NIH HHS/United States
- R01 NS056427/NS/NINDS NIH HHS/United States
- R0O NS057944-03/NS/NINDS NIH HHS/United States
- K99 NS057944/NS/NINDS NIH HHS/United States
- K99NS057944/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

