Cyclin-dependent kinase activity controls the onset of the HCMV lytic cycle
- PMID: 20844576
- PMCID: PMC2936547
- DOI: 10.1371/journal.ppat.1001096
Cyclin-dependent kinase activity controls the onset of the HCMV lytic cycle
Abstract
The onset of human cytomegalovirus (HCMV) lytic infection is strictly synchronized with the host cell cycle. Infected G0/G1 cells support viral immediate early (IE) gene expression and proceed to the G1/S boundary where they finally arrest. In contrast, S/G2 cells can be infected but effectively block IE gene expression and this inhibition is not relieved until host cells have divided and reentered G1. During latent infection IE gene expression is also inhibited, and for reactivation to occur this block to IE gene expression must be overcome. It is only poorly understood which viral and/or cellular activities maintain the block to cell cycle or latency-associated viral IE gene repression and whether the two mechanisms may be linked. Here, we show that the block to IE gene expression during S and G2 phase can be overcome by both genotoxic stress and chemical inhibitors of cellular DNA replication, pointing to the involvement of checkpoint-dependent signaling pathways in controlling IE gene repression. Checkpoint-dependent rescue of IE expression strictly requires p53 and in the absence of checkpoint activation is mimicked by proteasomal inhibition in a p53 dependent manner. Requirement for the cyclin dependent kinase (CDK) inhibitor p21 downstream of p53 suggests a pivotal role for CDKs in controlling IE gene repression in S/G2 and treatment of S/G2 cells with the CDK inhibitor roscovitine alleviates IE repression independently of p53. Importantly, CDK inhibiton also overcomes the block to IE expression during quiescent infection of NTera2 (NT2) cells. Thus, a timely block to CDK activity not only secures phase specificity of the cell cycle dependent HCMV IE gene expression program, but in addition plays a hitherto unrecognized role in preventing the establishment of a latent-like state.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


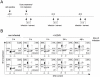

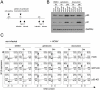
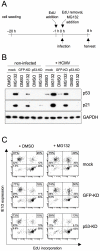






References
-
- Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA; 2007. pp. 2701–2772.
-
- Meier JL, Stinski MF. Major immediate-early enhancer and its gene products. In Cytomegaloviruses: Molecular Biology and Immunology ed. In: Reddehase M, editor. Norfolk: Caister Academic Press; 2006. pp. 151–166.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

