A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division
- PMID: 20844581
- PMCID: PMC2936552
- DOI: 10.1371/journal.ppat.1001094
A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division
Abstract
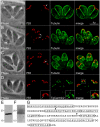
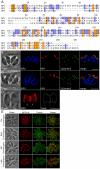
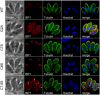
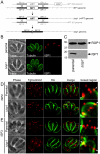
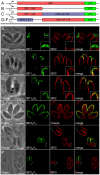
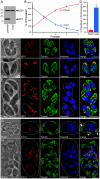
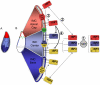
Apicomplexans employ a peripheral membrane system called the inner membrane complex (IMC) for critical processes such as host cell invasion and daughter cell formation. We have identified a family of proteins that define novel sub-compartments of the Toxoplasma gondii IMC. These IMC Sub-compartment Proteins, ISP1, 2 and 3, are conserved throughout the Apicomplexa, but do not appear to be present outside the phylum. ISP1 localizes to the apical cap portion of the IMC, while ISP2 localizes to a central IMC region and ISP3 localizes to a central plus basal region of the complex. Targeting of all three ISPs is dependent upon N-terminal residues predicted for coordinated myristoylation and palmitoylation. Surprisingly, we show that disruption of ISP1 results in a dramatic relocalization of ISP2 and ISP3 to the apical cap. Although the N-terminal region of ISP1 is necessary and sufficient for apical cap targeting, exclusion of other family members requires the remaining C-terminal region of the protein. This gate-keeping function of ISP1 reveals an unprecedented mechanism of interactive and hierarchical targeting of proteins to establish these unique sub-compartments in the Toxoplasma IMC. Finally, we show that loss of ISP2 results in severe defects in daughter cell formation during endodyogeny, indicating a role for the ISP proteins in coordinating this unique process of Toxoplasma replication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Hill DE, Chirukandoth S, Dubey JP. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev. 2005;6:41–61. - PubMed
-
- Sonda S, Hehl AB. Lipid biology of Apicomplexa: perspectives for new drug targets, particularly for Toxoplasma gondii. Trends Parasitol. 2006;22:41–47. - PubMed
-
- Gherardi A, Sarciron ME. Molecules targeting the purine salvage pathway in Apicomplexan parasites. Trends Parasitol. 2007;23:384–389. - PubMed
-
- McFadden GI, Roos DS. Apicomplexan plastids as drug targets. Trends Microbiol. 1999;7:328–333. - PubMed
-
- Keeling PJ, Burger G, Durnford DG, Lang BF, Lee RW, et al. The tree of eukaryotes. Trends Ecol Evol. 2005;20:670–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

