Connexin mediated cataract prevention in mice
- PMID: 20844585
- PMCID: PMC2936561
- DOI: 10.1371/journal.pone.0012624
Connexin mediated cataract prevention in mice
Abstract
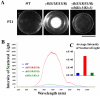
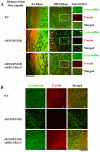
Cataracts, named for any opacity in the ocular lens, remain the leading cause of vision loss in the world. Non-surgical methods for cataract prevention are still elusive. We have genetically tested whether enhanced lens gap junction communication, provided by increased α3 connexin (Cx46) proteins expressed from α8(Kiα3) knock-in alleles in Gja8tm1(Gja3)Tww mice, could prevent nuclear cataracts caused by the γB-crystallin S11R mutation in CrygbS11R/S11R mice. Remarkably, homozygous knock-in α8(Kiα3/Kiα3) mice fully prevented nuclear cataracts, while single knock-in α8(Kiα3/-) allele mice showed variable suppression of nuclear opacities in CrygbS11R/S11R mutant mice. Cataract prevention was correlated with the suppression of many pathological processes, including crystallin degradation and fiber cell degeneration, as well as preservation of normal calcium levels and stable actin filaments in the lens. This work demonstrates that enhanced intercellular gap junction communication can effectively prevent or delay nuclear cataract formation and suggests that small metabolites transported through gap junction channels protect the stability of crystallin proteins and the cytoskeletal structures in the lens core. Thus, the use of an array of small molecules to promote lens homeostasis may become a feasible non-surgical approach for nuclear cataract prevention in the future.
Conflict of interest statement
Figures



References
-
- Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, et al. Age-related cataract. Lancet. 2005;365:599–609. - PubMed
-
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, et al. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. - PubMed
-
- Kuszak JR, Zoltoski RK, Sivertson C. Fibre cell organization in crystalline lenses. Exp Eye Res. 2004;78:673–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

