Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs
- PMID: 20844765
- PMCID: PMC2937036
- DOI: 10.1371/journal.pone.0012684
Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs
Abstract
Background: Several short antimicrobial peptides that are rich in tryptophan and arginine residues were designed with a series of simple modifications such as end capping and cyclization. The two sets of hexapeptides are based on the Trp- and Arg-rich primary sequences from the "antimicrobial centre" of bovine lactoferricin as well as an antimicrobial sequence obtained through the screening of a hexapeptide combinatorial library.
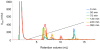
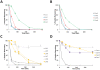
Methodology/principal findings: HPLC, mass spectrometry and antimicrobial assays were carried out to explore the consequences of the modifications on the serum stability and microbicidal activity of the peptides. The results show that C-terminal amidation increases the antimicrobial activity but that it makes little difference to its proteolytic degradation in human serum. On the other hand, N-terminal acetylation decreases the peptide activities but significantly increases their protease resistance. Peptide cyclization of the hexameric peptides was found to be highly effective for both serum stability and antimicrobial activity. However the two cyclization strategies employed have different effects, with disulfide cyclization resulting in more active peptides while backbone cyclization results in more proteolytically stable peptides. However, the benefit of backbone cyclization did not extend to longer 11-mer peptides derived from the same region of lactoferricin. Mass spectrometry data support the serum stability assay results and allowed us to determine preferred proteolysis sites in the peptides. Furthermore, isothermal titration calorimetry experiments showed that the peptides all had weak interactions with albumin, the most abundant protein in human serum.
Conclusions/significance: Taken together, the results provide insight into the behavior of the peptides in human serum and will therefore aid in advancing antimicrobial peptide design towards systemic applications.
Conflict of interest statement
Figures
References
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed
-
- Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. - PubMed
-
- Chan DI, Prenner EJ, Vogel HJ. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta. 2006;1758:1184–1202. - PubMed
-
- Park Y, Hahm KS. Antimicrobial peptides (AMPs): peptide structure and mode of action. J Biochem Mol Biol. 2005;38:507–516. - PubMed
-
- Kovalchuk LV, Gankovskaya LV, Gankovskaya OA, Lavrov VF. Herpes simplex virus: treatment with antimicrobial peptides. Adv Exp Med Biol. 2007;601:369–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

