cat-ELCCA: a robust method to monitor the fatty acid acyltransferase activity of ghrelin O-acyltransferase (GOAT)
- PMID: 20845345
- PMCID: PMC3485397
- DOI: 10.1002/anie.201003387
cat-ELCCA: a robust method to monitor the fatty acid acyltransferase activity of ghrelin O-acyltransferase (GOAT)
Abstract
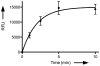
Assays armed with catalytic signal amplification have arisen as superior systems for ultrasensitive detection of analytes. Here we describe a conceptually new enzyme assay based on cat-ELISA, catalytic assay using enzyme-linked click chemistry assay (cat-ELCCA), where an enzyme-linked azide is utilized to arm the assay with catalytic fluorescence signal amplification. Using this assay technology, we have developed the first potentially high-throughput screen for the recently disclosed acyltransferase, ghrelin O-acyltransferase (GOAT).
Figures







Similar articles
-
A small molecule antagonist of ghrelin O-acyltransferase (GOAT).Chem Commun (Camb). 2011 Jul 14;47(26):7512-4. doi: 10.1039/c1cc11817j. Epub 2011 May 19. Chem Commun (Camb). 2011. PMID: 21594284
-
Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone.Cell. 2008 Feb 8;132(3):387-96. doi: 10.1016/j.cell.2008.01.017. Cell. 2008. PMID: 18267071
-
Ghrelin O-acyltransferase (GOAT), a specific enzyme that modifies ghrelin with a medium-chain fatty acid.J Biochem. 2016 Oct;160(4):189-194. doi: 10.1093/jb/mvw046. Epub 2016 Aug 3. J Biochem. 2016. PMID: 27489223 Review.
-
Structure-activity analysis of human ghrelin O-acyltransferase reveals chemical determinants of ghrelin selectivity and acyl group recognition.Biochemistry. 2015 Feb 3;54(4):1100-10. doi: 10.1021/bi5010359. Epub 2015 Jan 21. Biochemistry. 2015. PMID: 25562443
-
Ghrelin octanoylation by ghrelin O-acyltransferase: Unique protein biochemistry underlying metabolic signaling.Biochem Soc Trans. 2019 Feb 28;47(1):169-178. doi: 10.1042/BST20180436. Epub 2019 Jan 9. Biochem Soc Trans. 2019. PMID: 30626708 Review.
Cited by
-
Abdominal surgery inhibits circulating acyl ghrelin and ghrelin-O-acyltransferase levels in rats: role of the somatostatin receptor subtype 2.Am J Physiol Gastrointest Liver Physiol. 2011 Aug;301(2):G239-48. doi: 10.1152/ajpgi.00018.2011. Epub 2011 Jun 2. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21636529 Free PMC article.
-
Development and Implementation of an HTS-Compatible Assay for the Discovery of Selective Small-Molecule Ligands for Pre-microRNAs.SLAS Discov. 2018 Jan;23(1):47-54. doi: 10.1177/2472555217717944. Epub 2017 Jul 7. SLAS Discov. 2018. PMID: 28686847 Free PMC article.
-
Stress-related alterations of acyl and desacyl ghrelin circulating levels: mechanisms and functional implications.Peptides. 2011 Nov;32(11):2208-17. doi: 10.1016/j.peptides.2011.07.002. Epub 2011 Jul 12. Peptides. 2011. PMID: 21782868 Free PMC article. Review.
-
cat-ELCCA: catalyzing drug discovery through click chemistry.Chem Commun (Camb). 2018 Jun 19;54(50):6531-6539. doi: 10.1039/c8cc02332h. Chem Commun (Camb). 2018. PMID: 29781014 Free PMC article.
-
Ghrelin octanoylation by ghrelin O-acyltransferase: protein acylation impacting metabolic and neuroendocrine signalling.Open Biol. 2021 Jul;11(7):210080. doi: 10.1098/rsob.210080. Epub 2021 Jul 28. Open Biol. 2021. PMID: 34315274 Free PMC article. Review.
References
-
- Blaedel WJ, Boguslaski RC. Anal Chem. 1978;50:1026–1032.
-
- Zhu L, Lynch VM, Anslyn EV. Tetrahedron. 2004;60:7267–7275.
- Wu Q, Anslyn EV. J Am Chem Soc. 2004;126:14682–14683. - PubMed
- Wu Q, Anslyn EV. J Mater Chem. 2005;15:2815–2819.
- Houk RJT, Anslyn EV. New J Chem. 2007;31:729–735.
- Gianneschi NC, Nguyen ST, Mirkin CA. J Am Chem Soc. 2005;127:1644–1645. - PubMed
- Masar MS, III, Gianneschi NC, Oliveri CG, Stern CL, Nguyen ST, Mirkin CA. J Am Chem Soc. 2007;129:10149–10158. - PubMed
- Graf N, Göritz M, Krämer R. Angew Chem. 2006;118:4117–4119. - PubMed
- Angew Chem Int Ed. 2006;45:4013–4015. - PubMed
- Graf N, Krämer R. Chem Commun. 2006:4375–4376. - PubMed
- Song F, Garner AL, Koide K. J Am Chem Soc. 2007;129:12354–12355. - PubMed
- Garner AL, Koide K. J Am Chem Soc. 2008;130:16472–16473. - PubMed
- Garner AL, Song F, Koide K. J Am Chem Soc. 2009;131:5163–5171. - PubMed
-
- Varner AS, De Vos ML, Creaser SP, Peterson BR, Smith CD. Anal Biochem. 2002;308:160–167. - PubMed
- Varner AS, Ducker CE, Xia Z, Zhuang Y, De Vos ML, Smith CD. Biochem J. 2003;373:91–99. - PMC - PubMed
- Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, Rudel LL. J Lipid Res. 2004;45:378–386. - PubMed
- Ducker CE, Griffel LK, Smith RA, Keller SN, Zhuang Y, Xia Z, Diller JD, Smith CD. Mol Cancer Ther. 2006;5:1647–1659. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

