Formulation and immunogenicity studies of type III secretion system needle antigens as vaccine candidates
- PMID: 20845448
- PMCID: PMC3761878
- DOI: 10.1002/jps.22180
Formulation and immunogenicity studies of type III secretion system needle antigens as vaccine candidates
Abstract
Bacterial infections caused by Shigella flexneri, Salmonella typhimurium, and Burkholderia pseudomallei are currently difficult to prevent due to the lack of a licensed vaccine. Here we present formulation and immunogenicity studies for the three type III secretion system (TTSS) needle proteins MxiH(Δ5), PrgI(Δ5), and BsaL(Δ5) (each truncated by five residues at its C terminus) as potential candidates for vaccine development. These antigens are found to be thermally stabilized by the presence of carbohydrates and polyols. Additionally, all adsorb readily to aluminum hydroxide apparently through a combination of hydrogen bonds and/or Van der Waals forces. The interaction of these proteins with the aluminum-based adjuvant changes with time resulting in varying degrees of irreversible binding. Peptide maps of desorbed protein, however, suggest that chemical changes are not responsible for this irreversible association. The ability of MxiH(Δ5) and PrgI(Δ5) to elicit strong humoral immune responses was tested in a murine model. When administered intramuscularly as monomers, the needle components exhibited dose dependent immunogenic behavior. The polymerized version of MxiH was exceptionally immunogenic even at low doses. The responses of both monomeric and polymerized forms were boosted by adsorption to an aluminum salt adjuvant.
© 2010 Wiley-Liss, Inc. and the American Pharmacists Association
Figures

 ), PrgI Δ5 (
), PrgI Δ5 ( ) and BsaL Δ5 (
) and BsaL Δ5 ( ) at 0.2 mg/mL in isotonic 10 mM Histidine buffer at pH 6.0.
) at 0.2 mg/mL in isotonic 10 mM Histidine buffer at pH 6.0.
 ) and PrgI Δ5 (
) and PrgI Δ5 ( ).
).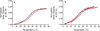
 ) and absence (-) of 10% Sucrose and 5% Dextrose.
) and absence (-) of 10% Sucrose and 5% Dextrose.


 ), Alhydrogel Control (
), Alhydrogel Control ( ), M1/M-His/P1 (
), M1/M-His/P1 ( ), M10/M-Al/P10 (
), M10/M-Al/P10 ( ), M50/N-His/P50 (
), M50/N-His/P50 ( ). N-Al/P-Soln (
). N-Al/P-Soln ( ).
).Similar articles
-
Formulation and immunogenicity of a potential multivalent type III secretion system-based protein vaccine.J Pharm Sci. 2010 Nov;99(11):4497-509. doi: 10.1002/jps.22195. J Pharm Sci. 2010. PMID: 20845449
-
SipD and IpaD induce a cross-protection against Shigella and Salmonella infections.PLoS Negl Trop Dis. 2020 May 28;14(5):e0008326. doi: 10.1371/journal.pntd.0008326. eCollection 2020 May. PLoS Negl Trop Dis. 2020. PMID: 32463817 Free PMC article.
-
Multicomponent Gold-Linked Glycoconjugate Vaccine Elicits Antigen-Specific Humoral and Mixed TH1-TH17 Immunity, Correlated with Increased Protection against Burkholderia pseudomallei.mBio. 2021 Jun 29;12(3):e0122721. doi: 10.1128/mBio.01227-21. Epub 2021 Jun 29. mBio. 2021. PMID: 34182777 Free PMC article.
-
Outer membrane protein A (OmpA) from Shigella flexneri 2a: a promising subunit vaccine candidate.Vaccine. 2013 Aug 12;31(36):3644-50. doi: 10.1016/j.vaccine.2013.05.100. Epub 2013 Jun 10. Vaccine. 2013. PMID: 23764536 Review.
-
Development of Burkholderia mallei and pseudomallei vaccines.Front Cell Infect Microbiol. 2013 Mar 11;3:10. doi: 10.3389/fcimb.2013.00010. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23508691 Free PMC article. Review.
Cited by
-
Förster resonance energy transfer (FRET) as a tool for dissecting the molecular mechanisms for maturation of the Shigella type III secretion needle tip complex.Int J Mol Sci. 2012 Nov 16;13(11):15137-61. doi: 10.3390/ijms131115137. Int J Mol Sci. 2012. PMID: 23203116 Free PMC article. Review.
-
Aluminum Adjuvants-'Back to the Future'.Pharmaceutics. 2023 Jul 4;15(7):1884. doi: 10.3390/pharmaceutics15071884. Pharmaceutics. 2023. PMID: 37514070 Free PMC article. Review.
-
The N terminus of type III secretion needle protein YscF from Yersinia pestis functions to modulate innate immune responses.Infect Immun. 2015 Apr;83(4):1507-22. doi: 10.1128/IAI.02687-14. Epub 2015 Feb 2. Infect Immun. 2015. PMID: 25644012 Free PMC article.
References
-
- Shigella. 2009 [cited 2009 February 19]; Available from: http://www.who.int/vaccine_research/diseases/shigella/en/
-
- World Health Organiation Drug-Resistant Salmonella. 2005 [cited 2009 February 19]; Available from: http://www.who.int/mediacentre/factsheets/fs139/en/print.html.
-
- Salmonella Saintpaul Outbreak. 2008 [cited 2009 February 19]; Available from: http://www.fda.gov/oc/opacom/hottopics/tomatoes.html.
-
- Peanut Product Recalls: Salmonella Typhimurium. 2008 [cited 2009 February 19]; Available from: http://www.fda.gov/oc/opacom/hottopics/Salmonellatyph.html.
-
- National Center for Zoonotic V-B, and Enteric Diseases (ZVED). Melioidosis. 2008 March 27 2008 [cited 2009 February 20]; Available from: http://www.cdc.gov/nczved/dfbmd/disease_listing/melioidosis_gi.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

