Current event-free survival after sequential tyrosine kinase inhibitor therapy for chronic myeloid leukemia
- PMID: 20845478
- PMCID: PMC4327987
- DOI: 10.1002/cncr.25604
Current event-free survival after sequential tyrosine kinase inhibitor therapy for chronic myeloid leukemia
Abstract
Background: Imatinib is an effective tyrosine kinase inhibitor (TKI) for patients with chronic myeloid leukemia (CML) in chronic phase (CP). Although some patients may fail on therapy with imatinib, effective salvage therapy is available with second-generation TKIs. Current measurement of efficacy for each therapy is judged by its individual impact on overall survival and event-free survival (EFS).
Methods: In total, 586 patients with CML in CP who received imatinib were included in this analysis in 2 cohorts: imatinib as front-line therapy (n = 281) or after failure on interferon-α (IFN-α) (n = 305). By accounting for successful salvage treatment (ie, regain of complete cytogenetic response), the current EFS (CEFS) rate was calculated to obtain a more accurate impression of the outcome of patients with CML who received treatment with sequential TKIs.
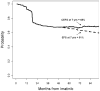
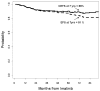
Results: For patients who received imatinib after failing on IFN-α, the 7-year EFS rate was 61%, whereas the CEFS rate was 69%. The 7-year EFS rate for patients who received imatinib as initial therapy was 81% compared with a 7-year CEFS rate of 88%.
Conclusions: CEFS provided a more accurate representation of the outcome of patients with CML in CP. These patients may frequently be treated successfully with subsequent TKIs after experiencing failure on the first TKI.
Copyright © 2010 American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
Dr. Cortes received research support from Novartis, Bristol-Myers Squibb, and Wyeth. Dr. Kantarjian received research support from Novartis and Bristol-Myers Squibb.
Figures
Comment in
-
Second-generation tyrosine kinase inhibitors in chronic myelogenous leukemia: before or after imatinib?Cancer. 2011 Jan 15;117(2):234-7. doi: 10.1002/cncr.25600. Epub 2010 Oct 26. Cancer. 2011. PMID: 21210471 No abstract available.
Similar articles
-
Survival advantage with imatinib mesylate therapy in chronic-phase chronic myelogenous ;eukemia (CML-CP) after IFN-alpha failure and in late CML-CP, comparison with historical controls.Clin Cancer Res. 2004 Jan 1;10(1 Pt 1):68-75. doi: 10.1158/1078-0432.ccr-1035-3. Clin Cancer Res. 2004. PMID: 14734453
-
Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-alpha treatment.Blood. 2008 Feb 1;111(3):1039-43. doi: 10.1182/blood-2007-07-103523. Epub 2007 Oct 11. Blood. 2008. PMID: 17932248 Clinical Trial.
-
EVI-1 oncogene expression predicts survival in chronic-phase CML patients resistant to imatinib treated with second-generation tyrosine kinase inhibitors.Blood. 2010 Dec 23;116(26):6014-7. doi: 10.1182/blood-2010-01-264234. Epub 2010 Sep 20. Blood. 2010. PMID: 20855863
-
Initial treatment for patients with CML.Hematology Am Soc Hematol Educ Program. 2009:453-60. doi: 10.1182/asheducation-2009.1.453. Hematology Am Soc Hematol Educ Program. 2009. PMID: 20008231 Review.
-
Targeted chronic myeloid leukemia therapy: Seeking a cure.Am J Health Syst Pharm. 2007 Dec 15;64(24 Suppl 15):S9-15. doi: 10.2146/ajhp070482. Am J Health Syst Pharm. 2007. PMID: 18056932 Review.
Cited by
-
Chronic myeloid leukemia: the race is yet to be won.CMAJ. 2012 May 15;184(8):857-8. doi: 10.1503/cmaj.111710. Epub 2012 Jan 23. CMAJ. 2012. PMID: 22271914 Free PMC article. No abstract available.
-
The evolution of treatment strategies for patients with chronic myeloid leukemia relapsing after allogeneic bone marrow transplant: can tyrosine kinase inhibitors replace donor lymphocyte infusions?Leuk Lymphoma. 2015 Jan;56(1):128-34. doi: 10.3109/10428194.2014.910868. Epub 2014 Jun 16. Leuk Lymphoma. 2015. PMID: 24712979 Free PMC article.
-
Discovery of N-(2-Acetamidobenzo[d]thiazol-6-yl)-2-phenoxyacetamide Derivatives as Novel Potential BCR-ABL1 Inhibitors Through Structure-Based Virtual Screening.Molecules. 2025 Feb 26;30(5):1065. doi: 10.3390/molecules30051065. Molecules. 2025. PMID: 40076290 Free PMC article.
-
Estimation of current cumulative incidence of leukaemia-free patients and current leukaemia-free survival in chronic myeloid leukaemia in the era of modern pharmacotherapy.BMC Med Res Methodol. 2011 Oct 11;11:140. doi: 10.1186/1471-2288-11-140. BMC Med Res Methodol. 2011. PMID: 21988861 Free PMC article.
-
Front-line and salvage therapies with tyrosine kinase inhibitors and other treatments in chronic myeloid leukemia.J Clin Oncol. 2011 Feb 10;29(5):524-31. doi: 10.1200/JCO.2010.31.3619. Epub 2011 Jan 10. J Clin Oncol. 2011. PMID: 21220597 Free PMC article. Review.
References
-
- Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. - PubMed
-
- Horner MJ, Ries LAG, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2006. Bethesda, Md: National Cancer Institute; [Accessed April 2010]. Based on November 2008 SEER data submission; posted to the SEER website 2009: http://seer.cancer.gov/csr/1975_2006.
-
- Guilhot F, Chastang C, Michallet M, et al. Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. French Chronic Myeloid Leukemia Study Group. N Engl J Med. 1997;337:223–229. - PubMed
-
- Bonifazi F, de Vivo A, Rosti G, et al. Chronic myeloid leukemia and interferon-alpha: a study of complete cytogenetic responders. Blood. 2001;98:3074–3081. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

