Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma
- PMID: 20845507
- PMCID: PMC2941063
- DOI: 10.3748/wjg.v16.i35.4410
Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma
Abstract
Aim: To evaluate the diagnostic value of glypican-3 (GPC3) in serum and liver for primary hepatocellular carcinoma (HCC).
Methods: Serum levels of GPC3 and α-fetoprotein (AFP) were measured in 75 patients with primary HCC and 32 patients with liver cirrhosis. Expression of GPC3 and AFP in 58 HCC and 12 cirrhotic specimens was detected with immunohistochemical staining.
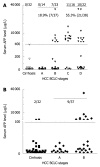
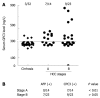
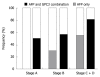
Results: When the cut-off value of serum GPC3 was set at 300 ng/L, its sensitivity and specificity for HCC were 47.0% and 93.5%, respectively. Among the 14 patients with HCC at stage according to the Barcelona Clinic Liver Cancer staging system, the serum GPC3 level was higher than 300 ng/L in 50% (7/14) patients, the serum AFP level was not ≥ 400 μg/L in any patient. Combined serum AFP and GPC3 significantly increased the sensitivity to the diagnosis of HCC. The GPC3 expression was detected in cytoplasm of HCC cells but not in hepatocytes and bile ducts of benign tumors. Among the 58 HCC patients, the GPC3 was expressed in 100% (28/28) patients with their serum AFP level ≥ 400 μg/L, and in 90% (27/30) patients with their AFP level < 400 μg/L, respectively. The GPC3 was weakly or negatively expressed in all paracarcinomatous and cirrhotic tissue samples. AFP positive HCC cells were only found in 1 out of the 58 HCC patients.
Conclusion: GPC3 protein is a sensitive and specific serum marker for diagnosis of early HCC. Its expression in liver tissues can be used to discriminate tumor cells from benign hepatic cells.
Figures


Similar articles
-
Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions.Arch Pathol Lab Med. 2008 Nov;132(11):1723-8. doi: 10.5858/132.11.1723. Arch Pathol Lab Med. 2008. PMID: 18976006
-
Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers.J Gastroenterol Hepatol. 2010 Jan;25(1):129-37. doi: 10.1111/j.1440-1746.2009.05988.x. Epub 2009 Sep 27. J Gastroenterol Hepatol. 2010. PMID: 19793164
-
Significance of Glypican-3 in Early Detection of Hepatocellular Carcinoma in Cirrhotic Patients.J Gastrointest Cancer. 2019 Sep;50(3):434-441. doi: 10.1007/s12029-018-0095-2. J Gastrointest Cancer. 2019. PMID: 29623600
-
Performance of Serum Glypican 3 in Diagnosis of Hepatocellular Carcinoma: A meta-analysis.Ann Hepatol. 2019 Jan-Feb;18(1):58-67. doi: 10.5604/01.3001.0012.7863. Ann Hepatol. 2019. PMID: 31113610 Review.
-
Usefulness of the novel oncofetal antigen glypican-3 for diagnosis of hepatocellular carcinoma and melanoma.BioDrugs. 2005;19(2):71-7. doi: 10.2165/00063030-200519020-00001. BioDrugs. 2005. PMID: 15807627 Review.
Cited by
-
Hepatocellular tumors: immunohistochemical analyses for classification and prognostication.Chin J Cancer Res. 2011 Dec;23(4):245-53. doi: 10.1007/s11670-011-0245-6. Chin J Cancer Res. 2011. PMID: 23359751 Free PMC article.
-
Clinical Impact of Circulated miR-1291 in Plasma of Patients with Liver Cirrhosis (LC) and Hepatocellular Carcinoma (HCC): Implication on Glypican-3 Expression.J Gastrointest Cancer. 2020 Mar;51(1):234-241. doi: 10.1007/s12029-019-00234-9. J Gastrointest Cancer. 2020. PMID: 31028536
-
Differential Expression of Glypican-3 and Insulin–Like Growth Factor-II mRNAs and Alpha-Fetoprotein and Ki-67 Markers in HCV Related Hepatocellular Carcinomas In Egyptian Patients.Asian Pac J Cancer Prev. 2017 Jan 1;18(1):121-127. doi: 10.22034/APJCP.2017.18.1.121. Asian Pac J Cancer Prev. 2017. PMID: 28240019 Free PMC article.
-
Idarubicin-loaded chitosan nanobubbles to improve survival and decrease drug side effects in hepatocellular carcinoma.Nanomedicine (Lond). 2025 Feb;20(3):255-270. doi: 10.1080/17435889.2025.2452154. Epub 2025 Jan 15. Nanomedicine (Lond). 2025. PMID: 39815170
-
Novel Nanotechnology Approaches to Overcome Drug Resistance in the Treatment of Hepatocellular Carcinoma: Glypican 3 as a Useful Target for Innovative Therapies.Int J Mol Sci. 2022 Sep 2;23(17):10038. doi: 10.3390/ijms231710038. Int J Mol Sci. 2022. PMID: 36077433 Free PMC article. Review.
References
-
- Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Shibata K, Ohta M, Kitano S. Short- and long-term outcomes after hepatic resection for hepatocellular carcinoma with concomitant esophageal varices in patients with cirrhosis. Ann Surg Oncol. 2008;15:1670–1676. - PubMed
-
- Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. - PubMed
-
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

