Polymorphic C-terminal beta-sheet interactions determine the formation of fibril or amyloid beta-derived diffusible ligand-like globulomer for the Alzheimer Abeta42 dodecamer
- PMID: 20847046
- PMCID: PMC2978638
- DOI: 10.1074/jbc.M110.133488
Polymorphic C-terminal beta-sheet interactions determine the formation of fibril or amyloid beta-derived diffusible ligand-like globulomer for the Alzheimer Abeta42 dodecamer
Abstract
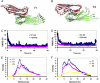
The relationship between amyloid deposition and cellular toxicity is still controversial. In addition to fibril-forming oligomers, other soluble Aβ forms (amyloid β-derived diffusible ligands (ADDLs)) were also suggested to form and to present different morphologies and mechanisms of toxicity. One ADDL type, the "globulomer," apparently forms independently of the fibril aggregation pathway. Even though many studies argue that such soluble Aβ oligomers are off fibril formation pathways, they may nonetheless share some structural similarity with protofibrils. NMR data of globulomer intermediates, "preglobulomers," suggested parallel in-register C-terminal β-sheets, with different N-terminal conformations. Based on experimental data, we computationally investigate four classes of Aβ dodecamers: fibril, fibril oligomer, prefibril/preglobulomer cluster, and globulomer models. Our simulations of the solvent protection of double-layered fibril and globulomer models reproduce experimental observations. Using a single layer Aβ fibril oligomer β-sheet model, we found that the C-terminal β-sheet in the fibril oligomer is mostly curved, preventing it from quickly forming a fibril and leading to its breaking into shorter pieces. The simulations also indicate that β-sheets packed orthogonally could be the most stable species for Aβ dodecamers. The major difference between fibril-forming oligomers and ADDL-like oligomers (globulomers) could be the exposure of Met-35 patches. Although the Met-35 patches are necessarily exposed in fibril-forming oligomers to allow their maturation into fibrils, the Met-35 patches in the globulomer are covered by other residues in the orthogonally packed Aβ peptides. Our results call attention to the possible existence of certain "critical intermediates" that can lead to both seeds and other soluble ADDL-like oligomers.
Figures





Similar articles
-
Abeta-globulomers are formed independently of the fibril pathway.Neurobiol Dis. 2008 May;30(2):212-20. doi: 10.1016/j.nbd.2008.01.010. Epub 2008 Feb 15. Neurobiol Dis. 2008. PMID: 18353662
-
Polymorphic structures of Alzheimer's β-amyloid globulomers.PLoS One. 2011;6(6):e20575. doi: 10.1371/journal.pone.0020575. Epub 2011 Jun 7. PLoS One. 2011. PMID: 21687730 Free PMC article.
-
Identifying the Template for Oligomer to Fibril Conversion for Amyloid-β (1-42) Oligomers using Hamiltonian Replica Exchange Molecular Dynamics.Chemphyschem. 2022 Dec 16;23(24):e202200393. doi: 10.1002/cphc.202200393. Epub 2022 Sep 16. Chemphyschem. 2022. PMID: 36052514
-
Aggregation and structure of amyloid β-protein.Neurochem Int. 2021 Dec;151:105208. doi: 10.1016/j.neuint.2021.105208. Epub 2021 Oct 13. Neurochem Int. 2021. PMID: 34655726 Review.
-
Molecular structures of amyloid and prion fibrils: consensus versus controversy.Acc Chem Res. 2013 Jul 16;46(7):1487-96. doi: 10.1021/ar300282r. Epub 2013 Jan 7. Acc Chem Res. 2013. PMID: 23294335 Free PMC article. Review.
Cited by
-
Structural differences between amyloid beta oligomers.Biochem Biophys Res Commun. 2016 Sep 2;477(4):700-705. doi: 10.1016/j.bbrc.2016.06.122. Epub 2016 Jun 27. Biochem Biophys Res Commun. 2016. PMID: 27363332 Free PMC article.
-
Structure of ring-shaped Aβ₄₂ oligomers determined by conformational selection.Sci Rep. 2016 Feb 12;6:21429. doi: 10.1038/srep21429. Sci Rep. 2016. PMID: 26868929 Free PMC article.
-
Unravelling γD-crystallin aggregation pathway to understand cataract formation using fluorescence correlation spectroscopy.Mol Vis. 2025 May 17;31:190-202. eCollection 2025. Mol Vis. 2025. PMID: 40606473 Free PMC article.
-
Comparative studies of disordered proteins with similar sequences: application to Aβ40 and Aβ42.Biophys J. 2013 Apr 2;104(7):1546-55. doi: 10.1016/j.bpj.2013.02.023. Biophys J. 2013. PMID: 23561531 Free PMC article.
-
Modeling amyloid-beta as homogeneous dodecamers and in complex with cellular prion protein.PLoS One. 2012;7(11):e49375. doi: 10.1371/journal.pone.0049375. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23145167 Free PMC article.
References
-
- Ahmad A., Millett I. S., Doniach S., Uversky V. N., Fink A. L. (2004) J. Biol. Chem. 279, 14999–15013 - PubMed
-
- Pitschke M., Prior R., Haupt M., Riesner D. (1998) Nat. Med. 4, 832–834 - PubMed
-
- Kirkitadze M. D., Bitan G., Teplow D. B. (2002) J. Neurosci. Res. 69, 567–577 - PubMed
-
- Walsh D. M., Selkoe D. J. (2004) Protein Pept. Lett. 11, 213–228 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

