Identification of the NC1 domain of {alpha}3 chain as critical for {alpha}3{alpha}4{alpha}5 type IV collagen network assembly
- PMID: 20847057
- PMCID: PMC3009915
- DOI: 10.1074/jbc.M110.149534
Identification of the NC1 domain of {alpha}3 chain as critical for {alpha}3{alpha}4{alpha}5 type IV collagen network assembly
Abstract
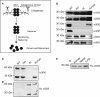
The network organization of type IV collagen consisting of α3, α4, and α5 chains in the glomerular basement membrane (GBM) is speculated to involve interactions of the triple helical and NC1 domain of individual α-chains, but in vivo evidence is lacking. To specifically address the contribution of the NC1 domain in the GBM collagen network organization, we generated a mouse with specific loss of α3NC1 domain while keeping the triple helical α3 chain intact by connecting it to the human α5NC1 domain. The absence of α3NC1 domain leads to the complete loss of the α4 chain. The α3 collagenous domain is incapable of incorporating the α5 chain, resulting in the impaired organization of the α3α4α5 chain-containing network. Although the α5 chain can assemble with the α1, α2, and α6 chains, such assembly is incapable of functionally replacing the α3α4α5 protomer. This novel approach to explore the assembly type IV collagen in vivo offers novel insights in the specific role of the NC1 domain in the assembly and function of GBM during health and disease.
Figures






References
-
- Kalluri R. (2003) Nat. Rev. Cancer 3, 422–433 - PubMed
-
- LeBleu S., MacDonald B., Kalluri R. (2007) Exp. Biol. Med. (Maywood) 232, 1121- 1129 - PubMed
-
- Hudson B. G., Reeders S. T., Tryggvason K. (1993) J. Biol. Chem. 268, 26033–26036 - PubMed
-
- Borza D. B., Bondar O., Ninomiya Y., Sado Y., Naito I., Todd P., Hudson B. G. (2001) J. Biol. Chem. 276, 28532–28540 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

