Human oocytes express ATP-sensitive K(+) channels
- PMID: 20847183
- PMCID: PMC2955558
- DOI: 10.1093/humrep/deq245
Human oocytes express ATP-sensitive K(+) channels
Abstract
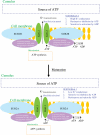
Background: ATP-sensitive K(+) (K(ATP)) channels link intracellular metabolism with membrane excitability and play crucial roles in cellular physiology and protection. The K(ATP) channel protein complex is composed of pore forming, Kir6.x (Kir6.1 or Kir6.2) and regulatory, SURx (SUR2A, SUR2B or SUR1), subunits that associate in different combinations. The objective of this study was to determine whether mammalian oocytes (human, bovine, porcine) express K(ATP) channels.
Methods: Supernumerary human oocytes at different stages of maturation were obtained from patients undergoing assisted conception treatments. Bovine and porcine oocytes in the germinal vesicle (GV) stage were obtained by aspirating antral follicles from abattoir-derived ovaries. The presence of mRNA for K(ATP) channel subunits was determined using real-time RT-PCR with primers specific for Kir6.2, Kir6.1, SUR1, SUR2A and SUR2B. To assess whether functional K(ATP) channels are present in human oocytes, traditional and perforated patch whole cell electrophysiology and immunoprecipitation/western blotting were used.
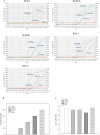
Results: Real-time PCR revealed that mRNA for Kir6.1, Kir6.2, SUR2A and SUR2B, but not SUR1, were present in human oocytes of different stages. Only SUR2B and Kir6.2 mRNAs were detected in GV stage bovine and porcine oocytes. Immunoprecipitation with SUR2 antibody and western blotting with Kir6.1 antibody identified bands corresponding to these subunits in human oocytes. In human oocytes, 2,4-dinitrophenol (400 µM), a metabolic inhibitor known to decrease intracellular ATP and activate K(ATP) channels, increased whole cell K(+) current. On the other hand, K(+) current induced by low intracellular ATP was inhibited by extracellular glibenclamide (30 µM), an oral antidiabetic known to block the opening of K(ATP) channels.
Conclusions: In conclusion, mammalian oocytes express K(ATP) channels. This opens a new avenue of research into the complex relationship between metabolism and membrane excitability in oocytes under different conditions, including conception.
Figures
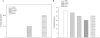



References
-
- Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of follicle development. Reproduction. 2001;121:647–653. - PubMed
-
- Boni R, Cocchia N, Silvestre F, Tortora G, Lorizio R, Tosti E. Juvenile and adult immature and in vitro matured ovine oocytes evaluated in relation to membrane electrical properties, calcium stores, IP3 sensitivity and apoptosis occurrence in cumulus cells. Mol Reprod Dev. 2008;75:1752–1760. - PubMed

