Roles of tetrahydrobiopterin in promoting tumor angiogenesis
- PMID: 20847284
- PMCID: PMC2966821
- DOI: 10.2353/ajpath.2010.100025
Roles of tetrahydrobiopterin in promoting tumor angiogenesis
Abstract
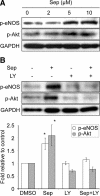
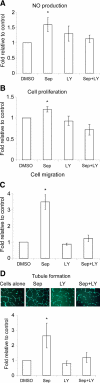
Nitric oxide (NO), which is derived from endothelial NO synthase (eNOS), provides crucial signals for angiogenesis in the tumor microenvironment. Tetrahydrobiopterin (BH4) is an absolute requirement for eNOS activity. In this study, we investigated whether this activation is both maintained by a wild-type Ras/phosphatidylinositol 3-kinase (PI3K)/Akt-positive feedback loop in endothelial cells and affects tumor angiogenesis. We found that supplementation of BH4 (via the pterin salvage pathway with Sep) increased Akt/eNOS phosphorylation in both human eNOS-transfected COS-7 cells and endothelial cells concomitant with increases in NO production, cell proliferation, migration, and tube formation. This augmentation was abrogated by a PI3K inhibitor. Sepiapterin (Sep) also increased GTP-bound wild-type Ras and PI3K/Akt/eNOS activation, which was prevented by the eNOS inhibitor, Nω-Nitro-L-arginine methyl ester (L-NAME). Furthermore, expression of GTP cyclohydrolase I (the rate-limiting enzyme in de novo BH4 synthesis) under doxycycline control potentiated in vivo tumorigenesis, tumor cell proliferation, as well as angiogenesis. Conversely, both switching off GTP cyclohydrolase I expression as well as inhibiting its enzymatic activity significantly decreased eNOS expression and tumor vascularization. This study demonstrates an important role for BH4 synthesis in angiogenesis by the activation of eNOS for NO production, which is maintained by a PI3K/Akt-positive feedback loop through effects on wild-type Ras in endothelial cells. Our findings suggest that BH4 synthesis may be a rational target for antiangiogenesis therapy for tumors.
Figures

References
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
-
- Heller R, Polack T, Grabner R, Till U. Nitric oxide inhibits proliferation of human endothelial cells via a mechanism independent of cGMP. Atherosclerosis. 1999;144:49–57. - PubMed
-
- Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. - PubMed
-
- Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

