Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria
- PMID: 20847295
- PMCID: PMC2947889
- DOI: 10.1073/pnas.1011099107
Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria
Abstract
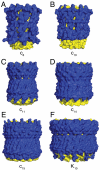
The catalytic domain of the F-ATPase in mitochondria protrudes into the matrix of the organelle, and is attached to the membrane domain by central and peripheral stalks. Energy for the synthesis of ATP from ADP and phosphate is provided by the transmembrane proton-motive-force across the inner membrane, generated by respiration. The proton-motive force is coupled mechanically to ATP synthesis by the rotation at about 100 times per second of the central stalk and an attached ring of c-subunits in the membrane domain. Each c-subunit carries a glutamate exposed around the midpoint of the membrane on the external surface of the ring. The rotation is generated by protonation and deprotonation successively of each glutamate. Each 360° rotation produces three ATP molecules, and requires the translocation of one proton per glutamate by each c-subunit in the ring. In fungi, eubacteria, and plant chloroplasts, ring sizes of c(10)-c(15) subunits have been observed, implying that these enzymes need 3.3-5 protons to make each ATP, but until now no higher eukaryote has been examined. As shown here in the structure of the bovine F(1)-c-ring complex, the c-ring has eight c-subunits. As the sequences of c-subunits are identical throughout almost all vertebrates and are highly conserved in invertebrates, their F-ATPases probably contain c(8)-rings also. Therefore, in about 50,000 vertebrate species, and probably in many or all of the two million invertebrate species, 2.7 protons are required by the F-ATPase to make each ATP molecule.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
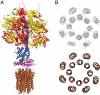
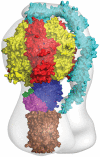

Comment in
-
ATP synthase: from sequence to ring size to the P/O ratio.Proc Natl Acad Sci U S A. 2010 Sep 28;107(39):16755-6. doi: 10.1073/pnas.1012260107. Epub 2010 Sep 21. Proc Natl Acad Sci U S A. 2010. PMID: 20858734 Free PMC article. No abstract available.
References
-
- Walker JE. ATP synthesis by rotary catalysis (Nobel lecture) Angewandte Chemie International Edition. 1998;37:5000–5011. - PubMed
-
- von Ballmoos C, Wiedenmann A, Dimroth P. Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem. 2009;78:649–672. - PubMed
-
- Junge W, Sielaff H, Engelbrecht S. Torque generation and elastic power transmission in the rotary FOF1-ATPase. Nature. 2009;459:364–370. - PubMed
-
- von Ballmoos C, Cook GM, Dimroth P. Unique rotary ATP synthase and its biological diversity. Annu Rev Biophys. 2008;37:43–64. - PubMed
-
- Abrahams JP, Leslie AGW, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–668. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

