Amyloid-β production via cleavage of amyloid-β protein precursor is modulated by cell density
- PMID: 20847415
- PMCID: PMC3272776
- DOI: 10.3233/JAD-2010-100816
Amyloid-β production via cleavage of amyloid-β protein precursor is modulated by cell density
Abstract
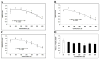
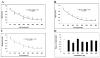
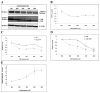
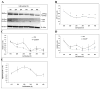
Mounting evidence suggests that Alzheimer's disease (AD) is caused by the accumulation of the small peptide, amyloid-β (Aβ), a proteolytic cleavage product of amyloid-β protein precursor (AβPP). Aβ is generated through a serial cleavage of AβPP by β- and γ-secretase. Aβ40 and Aβ42 are the two main components of amyloid plaques in AD brains, with Aβ42 being more prone to aggregation. AβPP can also be processed by α-secretase, which cleaves AβPP within the Aβ sequence, thereby preventing the generation of Aβ. Little is currently known regarding the effects of cell density on AβPP processing and Aβ generation. Here we assessed the effects of cell density on AβPP processing in neuronal and non-neuronal cell lines, as well as mouse primary cortical neurons. We found that decreased cell density significantly increases levels of Aβ40, Aβ42, total Aβ, and the ratio of Aβ42: Aβ40. These results also indicate that cell density is a significant modulator of AβPP processing. Overall, these findings carry profound implications for both previous and forthcoming studies aiming to assess the effects of various conditions and genetic/chemical factors, e.g., novel drugs on AβPP processing and Aβ generation in cell-based systems. Moreover, it is interesting to speculate whether cell density changes in vivo may also affect AβPP processing and Aβ levels in the AD brain.
Figures



References
-
- Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. - PubMed
-
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. - PubMed
-
- Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. - PubMed
-
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

