Central roles of NLRs and inflammasomes in viral infection
- PMID: 20847744
- PMCID: PMC3909537
- DOI: 10.1038/nri2851
Central roles of NLRs and inflammasomes in viral infection
Abstract
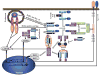
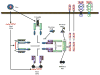
The immune response to viral infections is determined by a complex interplay between the pathogen and the host. Innate immune cells express a set of cytosolic sensors to detect viral infection. Recognition by these sensors induces the production of type I interferons and the assembly of inflammasome complexes that activate caspase-1, leading to production of interleukin-1β (IL-1β) and IL-18. Here, I discuss recent progress in our understanding of the central roles of NOD-like receptors (NLRs) and inflammasomes in the immune response during viral infections. This information will improve our understanding of host defence mechanisms against viruses and provide new avenues for interfering in the pathogenesis of infectious diseases.
Conflict of interest statement
The author declares no competing financial interests.
Figures
References
-
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
-
- Harton JA, Linhoff MW, Zhang J, Ting JP. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J Immunol. 2002;169:4088–93. - PubMed
-
- Inohara N, Nunez G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene. 2001;20:6473–81. References 3 and 4 report the discovery of the NLR family. - PubMed
-
- Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

