Assessment of acute and chronic pharmacological effects on energy expenditure and macronutrient oxidation in humans: responses to ephedrine
- PMID: 20847897
- PMCID: PMC2931375
- DOI: 10.1155/2011/210484
Assessment of acute and chronic pharmacological effects on energy expenditure and macronutrient oxidation in humans: responses to ephedrine
Abstract
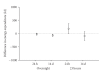
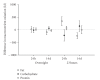
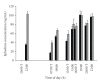
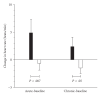
Evidence of active brown adipose tissue in human adults suggests that this may become a pharmacological target to induce negative energy balance. We have explored whole-body indirect calorimetry to detect the metabolic effects of thermogenic drugs through administration of ephedrine hydrochloride and have assessed ephedrine's merits as a comparator compound in the evaluation of novel thermogenic agents. Volunteers randomly given ephedrine hydrochloride 15 mg QID (n = 8) or placebo (n = 6) were studied at baseline and after 1-2 and 14-15 days of treatment. We demonstrate that overnight or 23-hour, 2% energy expenditure (EE) and 5% fat (FO) or CHO oxidation effects are detectable both acutely and over 14 days. Compared to placebo, ephedrine increased EE and FO rates overnight (EE 63 kJ day 2, EE 105 kJ, FO 190 kJ, day 14), but not over 23 h. We conclude that modest energy expenditure and fat oxidation responses to pharmacological interventions can be confidently detected by calorimetry in small groups. Ephedrine should provide reliable data against which to compare novel thermogenic compounds.
Figures
Similar articles
-
Acute effect of ephedrine on 24-h energy balance.Clin Sci (Lond). 1999 May;96(5):483-91. Clin Sci (Lond). 1999. PMID: 10209080 Clinical Trial.
-
Chronic ephedrine administration decreases brown adipose tissue activity in a randomised controlled human trial: implications for obesity.Diabetologia. 2015 May;58(5):1045-54. doi: 10.1007/s00125-015-3543-6. Epub 2015 Mar 1. Diabetologia. 2015. PMID: 25725625 Clinical Trial.
-
Bioactive food stimulants of sympathetic activity: effect on 24-h energy expenditure and fat oxidation.Eur J Clin Nutr. 2005 Jun;59(6):733-41. doi: 10.1038/sj.ejcn.1602121. Eur J Clin Nutr. 2005. PMID: 15870822 Clinical Trial.
-
Pharmacology of thermogenic drugs.Am J Clin Nutr. 1992 Jan;55(1 Suppl):246S-248S. doi: 10.1093/ajcn/55.1.246s. Am J Clin Nutr. 1992. PMID: 1345887 Review.
-
Brown adipose tissue in the treatment of obesity and diabetes: Are we hot enough?J Diabetes Investig. 2011 Oct 7;2(5):341-50. doi: 10.1111/j.2040-1124.2011.00158.x. J Diabetes Investig. 2011. PMID: 24843510 Free PMC article. Review.
Cited by
-
Ephedrine activates brown adipose tissue in lean but not obese humans.Diabetologia. 2013 Jan;56(1):147-55. doi: 10.1007/s00125-012-2748-1. Epub 2012 Oct 13. Diabetologia. 2013. PMID: 23064293 Clinical Trial.
References
-
- Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. The New England Journal of Medicine. 2005;353(20):2111–2120. - PubMed
-
- Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technology Assessment. 2004;8(21):1–182. - PubMed
-
- Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Annals of Internal Medicine. 2007;147(1):41–50. - PubMed
-
- Astrup A, Breum L, Toubro S, Hein P, Quaade F. The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in obese subjects on an energy restricted diet. A double blind trial. International Journal of Obesity and Related Metabolic Disorders. 1992;16(4):269–277. - PubMed
-
- Astrup A, Madsbad S, Breum L, Jensen TJ, Kroustrup JP, Larsen TM. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. The Lancet. 2008;372(9653):1906–1913. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

