Revisiting the Kinnel-Scheuer hypothesis for the biosynthesis of palau'amine
- PMID: 20848010
- PMCID: PMC2999656
- DOI: 10.1039/c0cc02214d
Revisiting the Kinnel-Scheuer hypothesis for the biosynthesis of palau'amine
Abstract
We propose herein an alternative biosynthetic pathway for palau'amine in order to resolve the stereochemical issue from the original Kinnel-Scheuer hypothesis. Furthermore, we use this revised hypothesis as a guide toward the laboratory synthesis of palau'amine.
Figures
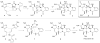

References
-
- Gaich T, Baran PS. J Org Chem. 2010;75:4657–4673. - PubMed
- Heasley B. Eur J Org Chem. 2009:1477–1489.
- Arndt HD, Riedrich M. Angew Chem, Int Ed. 2008;47:4785–4788. - PubMed
- Köck M, Grube A, Seiple IB, Baran PS. Angew Chem, Int Ed. 2007;46:6586–6594. - PubMed
- Jacquot DEN, Lindel T. Curr Org Chem. 2005;9:1551–1565.
- Hoffmann H, Lindel T. Synthesis. 2003:1753–1783.
-
-
For example: Overman LE, Rogers BN, Tellew JE, Trenkle WC. J Am Chem Soc. 1997;119:7159–7160.Starr JT, Koch G, Carreira EM. J Am Chem Soc. 2000;122:8793–8794.Lovely CJ, Du H, Dias HVR. Org Lett. 2001;3:1319–1322.Dilley AS, Romo D. Org Lett. 2001;3:1535–1538.Kawasaki I, Sakaguchi N, Fukushima N, Fujioka N, Nikaido F, Yamashita M, Ohta S. Tetrahedron Lett. 2002;43:4377–4380.Koenig SG, Miller SM, Leonard KA, Löwe RS, Chen BC, Austin DJ. Org Lett. 2003;5:2203–2206.Baran PS, Zografos AL, O’Malley DP. J Am Chem Soc. 2004;126:3726–3727.Baran PS, O’Malley DP, Zografos AL. Angew Chem, Int Ed. 2004;43:2674–2677.Birman VB, Jiang XT. Org Lett. 2004;6:2369–2371.Garrido-Hernandez H, Nakadai M, Vimolratana M, Li Q, Doundoulakis T, Harran PG. Angew Chem, Int Ed. 2005;44:765–769.Kawasaki I, Sakaguchi N, Khadeer A, Yamashita M, Ohta S. Tetrahedron. 2006;62:10182–10192.Bultman MS, Ma J, Gin DY. Angew Chem, Int Ed. 2008;47:6821–6824.Hudon J, Cernak TA, Ashenhurst JA, Gleason JL. Angew Chem, Int Ed. 2008;47:8885–8888.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

