Convergent genesis of an adult neural crest-like dermal stem cell from distinct developmental origins
- PMID: 20848654
- PMCID: PMC3087810
- DOI: 10.1002/stem.525
Convergent genesis of an adult neural crest-like dermal stem cell from distinct developmental origins
Abstract
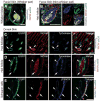
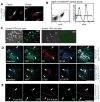
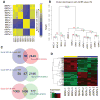
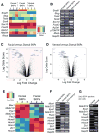
Skin-derived precursors (SKPs) are multipotent dermal stem cells that reside within a hair follicle niche and that share properties with embryonic neural crest precursors. Here, we have asked whether SKPs and their endogenous dermal precursors originate from the neural crest or whether, like the dermis itself, they originate from multiple developmental origins. To do this, we used two different mouse Cre lines that allow us to perform lineage tracing: Wnt1-cre, which targets cells deriving from the neural crest, and Myf5-cre, which targets cells of a somite origin. By crossing these Cre lines to reporter mice, we show that the endogenous follicle-associated dermal precursors in the face derive from the neural crest, and those in the dorsal trunk derive from the somites, as do the SKPs they generate. Despite these different developmental origins, SKPs from these two locations are functionally similar, even with regard to their ability to differentiate into Schwann cells, a cell type only thought to be generated from the neural crest. Analysis of global gene expression using microarrays confirmed that facial and dorsal SKPs exhibit a very high degree of similarity, and that they are also very similar to SKPs derived from ventral dermis, which has a lateral plate origin. However, these developmentally distinct SKPs also retain differential expression of a small number of genes that reflect their developmental origins. Thus, an adult neural crest-like dermal precursor can be generated from a non-neural crest origin, a finding with broad implications for the many neuroendocrine cells in the body.
Copyright © 2010 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures



Similar articles
-
A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin.Stem Cells. 2008 Jan;26(1):163-72. doi: 10.1634/stemcells.2007-0281. Epub 2007 Sep 27. Stem Cells. 2008. PMID: 17901404
-
A dermal niche for multipotent adult skin-derived precursor cells.Nat Cell Biol. 2004 Nov;6(11):1082-93. doi: 10.1038/ncb1181. Nat Cell Biol. 2004. PMID: 15517002
-
[Research progress of skin-derived precursor cells].Nan Fang Yi Ke Da Xue Xue Bao. 2017 Mar 20;37(3):420-422. doi: 10.3969/j.issn.1673-4254.2017.03.26. Nan Fang Yi Ke Da Xue Xue Bao. 2017. PMID: 28377365 Free PMC article. Review. Chinese.
-
Isolation and differentiation of hair follicle-derived dermal precursors.Methods Mol Biol. 2013;989:247-63. doi: 10.1007/978-1-62703-330-5_19. Methods Mol Biol. 2013. PMID: 23483400
-
Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential.Philos Trans R Soc Lond B Biol Sci. 2008 Jan 12;363(1489):185-98. doi: 10.1098/rstb.2006.2020. Philos Trans R Soc Lond B Biol Sci. 2008. PMID: 17282990 Free PMC article. Review.
Cited by
-
Evolution of the sheep coat: the impact of domestication on its structure and development.Genet Res (Camb). 2020 Jun 10;102:e4. doi: 10.1017/S0016672320000063. Genet Res (Camb). 2020. PMID: 32517826 Free PMC article.
-
Clonal growth of dermal papilla cells in hydrogels reveals intrinsic differences between Sox2-positive and -negative cells in vitro and in vivo.J Invest Dermatol. 2012 Apr;132(4):1084-93. doi: 10.1038/jid.2011.428. Epub 2011 Dec 22. J Invest Dermatol. 2012. PMID: 22189784 Free PMC article.
-
The Ocular Neural Crest: Specification, Migration, and Then What?Front Cell Dev Biol. 2020 Dec 23;8:595896. doi: 10.3389/fcell.2020.595896. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33425902 Free PMC article. Review.
-
Specific and spatial labeling of P0-Cre versus Wnt1-Cre in cranial neural crest in early mouse embryos.Genesis. 2017 Jun;55(6):10.1002/dvg.23034. doi: 10.1002/dvg.23034. Epub 2017 Apr 18. Genesis. 2017. PMID: 28371069 Free PMC article.
-
Regulation of skin aging and heart development by TAp63.Cell Death Differ. 2012 Feb;19(2):186-93. doi: 10.1038/cdd.2011.181. Epub 2011 Dec 9. Cell Death Differ. 2012. PMID: 22158419 Free PMC article. Review.
References
-
- Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. - PubMed
-
- Lavoie JF, Biernaskie JA, Chen Y, et al. Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem Cells Dev. 2009;18:893–906. - PubMed
-
- Biernaskie JA, McKenzie IA, Toma JG, et al. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc. 2006;1:2803–2812. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

