A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer
- PMID: 20849361
- PMCID: PMC3760671
- DOI: 10.3109/14653249.2010.515582
A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer
Abstract
Background: Natural killer (NK) cells derived from patients with cancer exhibit diminished cytotoxicity compared with NK cells from healthy individuals. We evaluated the tumor response and in vivo expansion of allogeneic NK cells in recurrent ovarian and breast cancer.
Methods: Patients underwent a lymphodepleting preparative regimen: fludarabine 25 mg/m(2) × 5 doses, cyclophosphamide 60 mg/kg × 2 doses, and, in seven patients, 200 cGy total body irradiation (TBI) to increase host immune suppression. An NK cell product, from a haplo-identical related donor, was incubated overnight in 1000 U/mL interleukin (IL)-2 prior to infusion. Subcutaneous IL-2 (10 MU) was given three times/week × 6 doses after NK cell infusion to promote expansion, defined as detection of ≥100 donor-derived NK cells/μL blood 14 days after infusion, based on molecular chimerism and flow cytometry.
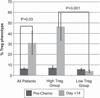
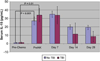
Results: Twenty (14 ovarian, 6 breast) patients were enrolled. The median age was 52 (range 30-65) years. Mean NK cell dose was 2.16 × 10(7)cells/kg. Donor DNA was detected 7 days after NK cell infusion in 9/13 (69%) patients without TBI and 6/7 (85%) with TBI. T-regulatory cells (Treg) were elevated at day +14 compared with pre-chemotherapy (P = 0.03). Serum IL-15 levels increased after the preparative regimen (P = <0.001). Patients receiving TBI had delayed hematologic recovery (P = 0.014). One patient who was not evaluable had successful in vivo NK cell expansion.
Conclusions: Adoptive transfer of haplo-identical NK cells after lymphodepleting chemotherapy is associated with transient donor chimerism and may be limited by reconstituting recipient Treg cells. Strategies to augment in vivo NK cell persistence and expansion are needed.
Conflict of interest statement
Figures

References
-
- Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–2229. - PubMed
-
- Miller JS, Verfaillie C, McGlave P. The generation of human natural killer cells from CD34+/DR-primitive progenitors in long-term bone marrow culture. Blood. 1992;80:2182–2187. - PubMed
-
- Pierson B, Miller J, Verfaillie B, McGlave P, Hu W-S. Population dynamics of human activated killer cells in culture. Biotech Bioeng. 1994;43:685–692. - PubMed
-
- Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83:2594–2601. - PubMed
-
- Miller JS, Klingsporn S, Lund J, Perry EH, Verfaillie C, McGlave P. Large scale ex vivo expansion and activation of human natural killer cells for autologous therapy. Bone Marrow Transplant. 1994;14:555–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

