p53 prevents progression of nevi to melanoma predominantly through cell cycle regulation
- PMID: 20849464
- PMCID: PMC3137930
- DOI: 10.1111/j.1755-148X.2010.00773.x
p53 prevents progression of nevi to melanoma predominantly through cell cycle regulation
Abstract
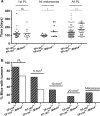
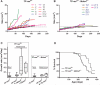
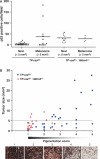
p53 is the central member of a critical tumor suppressor pathway in virtually all tumor types, where it is silenced mainly by missense mutations. In melanoma, p53 predominantly remains wild type, thus its role has been neglected. To study the effect of p53 on melanocyte function and melanomagenesis, we crossed the ‘high-p53’Mdm4+/− mouse to the well-established TP-ras0/+ murine melanoma progression model. After treatment with the carcinogen dimethylbenzanthracene (DMBA), TP-ras0/+ mice on the Mdm4+/− background developed fewer tumors with a delay in the age of onset of melanomas compared to TP-ras0/+ mice. Furthermore, we observed a dramatic decrease in tumor growth, lack of metastasis with increased survival of TP-ras0/+: Mdm4+/− mice. Thus, p53 effectively prevented the conversion of small benign tumors to malignant and metastatic melanoma. p53 activation in cultured primary melanocyte and melanoma cell lines using Nutlin-3, a specific Mdm2 antagonist, supported these findings. Moreover, global gene expression and network analysis of Nutlin-3-treated primary human melanocytes indicated that cell cycle regulation through the p21WAF1/CIP1 signaling network may be the key anti-melanomagenic activity of p53.
Figures





References
-
- Abdel-Malek ZA, Kadekaro AL, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010;23:171–186. - PubMed
-
- Albino AP, Vidal MJ, Mcnutt NS, Shea CR, Prieto VG, Nanus DM, Palmer JM, Hayward NK. Mutation and expression of the p53 gene in human malignant melanoma. Melanoma Res. 1994;4:35–45. - PubMed
-
- Bae I, Smith ML, Sheikh MS, Zhan Q, Scudiero DA, Friend SH, O'connor PM, Fornace AJ., Jr An abnormality in the p53 pathway following gamma-irradiation in many wild-type p53 human melanoma lines. Cancer Res. 1996;56:840–847. - PubMed
-
- Bandyopadhyay D, Gatza C, Donehower LA, Medrano EE. Analysis of cellular senescence in culture in vivo: the senescence-associated beta-galactosidase assay. Curr. Protoc. Cell Biol. 2005 Chapter 18, Unit 18.9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

