Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs
- PMID: 20849469
- PMCID: PMC11158092
- DOI: 10.1111/j.1349-7006.2010.01723.x
Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs
Abstract
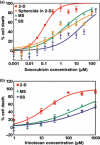
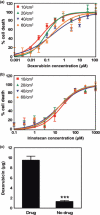
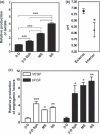
Development of cancer therapeutics requires a thorough evaluation of drug efficacy in vitro before animal testing and subsequent clinical trials. Three-dimensional (3-D) in vitro models have therefore been investigated for drug screening. In this study, we have developed a novel in vitro model in which multicellular aggregates, or spheroids, were incorporated into 3-D porous scaffolds. Drug resistance assays showed that spheroid-seeded scaffolds have much higher drug resistance than monolayer cultures, spheroids on flat substrates, or scaffolds seeded with dispersed cells. Furthermore, spheroid-seeded scaffolds demonstrated higher lactate production leading to acidosis, and higher expression of angiogenic factors. These data suggest that the spheroid-seeded 3-D scaffolds might serve as a useful in vitro system for screening cancer therapeutics.
© 2010 Japanese Cancer Association.
Figures

References
-
- Griffith L, Swartz M. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 2006; 7: 211–24. - PubMed
-
- Newman MJ, Rodarte JC, Benbatoul KD et al. Discovery and characterization of OC144‐093, a novel inhibitor of P‐glycoprotein‐mediated multidrug resistance. Cancer Res 2000; 60: 2964–72. - PubMed
-
- Torrance CJ, Agrawal V, Vogelstein B, Kinzler KW. Use of isogenic human cancer cells for high‐throughput screening and drug discovery. Nat Biotechnol 2001; 19: 940–5. - PubMed
-
- Holtfreter J. A study of the mechanics of gastrulation. J Exp Zool 1944; 95: 171–212.
-
- Moscona A. Cell suspensions from organ rudiments of chick embryos. Exp Cell Res 1952; 3: 535–9.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

