A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma
- PMID: 20849846
- PMCID: PMC3128181
- DOI: 10.1016/j.exer.2010.09.005
A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma
Abstract
This article is dedicated to Rosario Hernandez for her warm support of my own work and her genuine enthusiasm for the work of her colleagues throughout her career. I first met Rosario as a research fellow in Harry Quigley's laboratory between 1991 and 1993. Along with Harry, John Morrison, Elaine Johnson, Abe Clark, Colm O'Brien and many others, Rosario's work has provided lamina cribrosa astrocyte cellular mechanisms that are biomechanically plausible and in so doing provided credibility to early notions of the optic nerve head (ONH) as a biomechanical structure. We owe a large intellectual debt to Rosario for her dogged persistence in the characterization of the ONH astrocyte and lamina cribrosacyte in age and disease. Two questions run through her work and remain of central importance today. First, how do astrocytes respond to and alter the biomechanical environment of the ONH and the physiologic stresses created therein? Second, how do these physiologic demands on the astrocyte influence their ability to deliver the support to retinal ganglion cell axon transport and flow against the translaminar pressure gradient? The purpose of this article is to summarize what is known about the biomechanical determinants of retinal ganglion cell axon physiology within the ONH in the optic neuropathy of aging and Glaucoma. My goal is to provide a biomechanical framework for this discussion. This framework assumes that the ONH astrocytes and glia fundamentally support and influence both the lamina cribrosa extracellular matrix and retinal ganglion cell axon physiology. Rosario Hernandez was one of the first investigators to recognize the implications of this unique circumstance. Many of the ideas contained herein have been initially presented within or derived from her work (Hernandez, M.R., 2000. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 19, 297-321.; Hernandez, M.R., Pena, J.D., 1997. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol. 115, 389-395.).
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


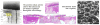


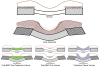


References
-
- Agapova OA, et al. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003;967:132–143. - PubMed
-
- Agapova OA, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia. 2001;33:205–216. - PubMed
-
- Agoumi YS, et al. Laminar and Prelaminar Tissue Displacement During Intraocular Pressure Elevation in Glaucoma Patients and Healthy Controls. Ophthalmology. 2010 In press. - PubMed
-
- Albon J, et al. Connective Tissue Structure of the Tree Shrew Optic Nerve and Associated Ageing Changes. Invest Ophthalmol Vis Sci. 2007;48:2134–2144. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

