Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization
- PMID: 20850011
- PMCID: PMC4115189
- DOI: 10.1016/j.cell.2010.08.017
Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization
Abstract
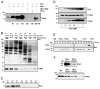
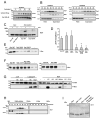
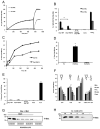
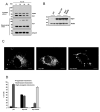
In response to many apoptotic stimuli, oligomerization of Bax is essential for mitochondrial outer membrane permeabilization and the ensuing release of cytochrome c. These events are accompanied by mitochondrial fission that appears to require Drp1, a large GTPase of the dynamin superfamily. Loss of Drp1 leads to decreased cytochrome c release by a mechanism that is poorly understood. Here we show that Drp1 stimulates tBid-induced Bax oligomerization and cytochrome c release by promoting tethering and hemifusion of membranes in vitro. This function of Drp1 is independent of its GTPase activity and relies on arginine 247 and the presence of cardiolipin in membranes. In cells, overexpression of Drp1 R247A/E delays Bax oligomerization and cell death. Our findings uncover a function of Drp1 and provide insight into the mechanism of Bax oligomerization.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Ardail D, Privat JP, Egret-Charlier M, Levrat C, Lerme F, Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. J Biol Chem. 1990;265:18797–18802. - PubMed
-
- Arnoult D, Rismanchi N, Grodet A, Roberts RG, Seeburg DP, Estaquier J, Sheng M, Blackstone C. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol. 2005;15:2112–2118. - PubMed
-
- Basanez G, Fidelio GD, Goni FM, Maggio B, Alonso A. Dual inhibitory effect of gangliosides on phospholipase C-promoted fusion of lipidic vesicles. Biochemistry. 1996;35:7506–7513. - PubMed
-
- Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem. 2002;277:49360–49365. - PubMed
-
- Bellot G, Cartron PF, Er E, Oliver L, Juin P, Armstrong LC, Bornstein P, Mihara K, Manon S, Vallette FM. TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax. Cell Death Differ. 2006;14:785–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

