Evaluation of the D3 dopamine receptor selective agonist/partial agonist PG01042 on L-dopa dependent animal involuntary movements in rats
- PMID: 20850462
- PMCID: PMC3820002
- DOI: 10.1016/j.neuropharm.2010.09.011
Evaluation of the D3 dopamine receptor selective agonist/partial agonist PG01042 on L-dopa dependent animal involuntary movements in rats
Abstract
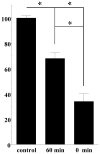
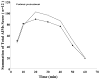
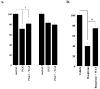
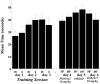
The substituted 4-phenylpiperazine D3 dopamine receptor selective antagonist PG01037 ((E)-N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)but-2-enyl)-4-(pyridin-2-yl)benzamide) was reported to attenuate L-dopa-associated abnormal involuntary movements (AIMs) in unilaterally lesioned rats, a model of L-dopa-dependent dyskinesia in patients with Parkinson's Disease (Kumar et al., 2009a). We now report that PG01042 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-3-yl)benzamide), which is a D3 dopamine receptor selective agonist for adenylyl cyclase inhibition and a partial agonist for mitogenesis, is also capable of attenuating AIMs scores. The intrinsic activity of PG01037 and PG01042 were determined using a) a forskolin-dependent adenylyl cyclase inhibition assay and b) an assay for agonist-associated mitogenesis. It was observed that the in vivo efficacy of PG01042 increased when administered by intraperitoneal (i.p.) injection simultaneously with L-dopa/benserazide (8 mg/kg each), as compared to a 60 min or 30 min pretreatment. PG01042 was found to attenuate AIM scores in these animals in a dose dependent manner. While PG01042 did not effectively inhibit SKF 81297-dependent AIMs, it inhibited apomorphine-dependent AIM scores. Rotarod studies indicate that PG01042 at a dose of 10 mg/kg did not adversely affect motor coordination of the unilaterally lesioned rats. Evaluation of lesioned rats using a cylinder test behavioral paradigm indicated that PG01042 did not dramatically attenuate the beneficial effects of L-dopa. These studies and previously published studies suggest that both D3 dopamine receptor selective antagonists, partial agonists and agonists, as defined by an adenylyl cyclase inhibition assay and a mitogenic assay, are pharmacotherapeutic candidates for the treatment of L-dopa-associated dyskinesia in patients with Parkinson's Disease.
Copyright © 2010. Published by Elsevier Ltd.
Figures


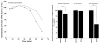

Similar articles
-
Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-dopa-dependent abnormal involuntary movements in rats.Neuropharmacology. 2009 May-Jun;56(6-7):944-55. doi: 10.1016/j.neuropharm.2009.01.020. Epub 2009 Feb 5. Neuropharmacology. 2009. PMID: 19371585 Free PMC article.
-
Evaluation of D2 and D3 dopamine receptor selective compounds on L-dopa-dependent abnormal involuntary movements in rats.Neuropharmacology. 2009 May-Jun;56(6-7):956-69. doi: 10.1016/j.neuropharm.2009.01.019. Epub 2009 Feb 5. Neuropharmacology. 2009. PMID: 19371586 Free PMC article.
-
Pharmacological modulation of abnormal involuntary DOI-induced head twitch response movements in male DBA/2J mice: II. Effects of D3 dopamine receptor selective compounds.Neuropharmacology. 2015 Jun;93:179-90. doi: 10.1016/j.neuropharm.2014.10.030. Epub 2015 Feb 17. Neuropharmacology. 2015. PMID: 25698528
-
Role of dopamine D3 and serotonin 5-HT 1A receptors in L: -DOPA-induced dyskinesias and effects of sarizotan in the 6-hydroxydopamine-lesioned rat model of Parkinson's disease.J Neural Transm (Vienna). 2011 Dec;118(12):1733-42. doi: 10.1007/s00702-010-0571-8. Epub 2011 Jan 21. J Neural Transm (Vienna). 2011. PMID: 21253782
-
Dopamine receptor partial agonists and addiction.Eur J Pharmacol. 2015 Apr 5;752:112-5. doi: 10.1016/j.ejphar.2015.02.025. Epub 2015 Feb 25. Eur J Pharmacol. 2015. PMID: 25724788 Review.
Cited by
-
Severity of Dyskinesia and D3R Signaling Changes Induced by L-DOPA Treatment of Hemiparkinsonian Rats Are Features Inherent to the Treated Subjects.Biomolecules. 2019 Sep 1;9(9):431. doi: 10.3390/biom9090431. Biomolecules. 2019. PMID: 31480516 Free PMC article.
-
Neurobiological and Pharmacological Perspectives of D3 Receptors in Parkinson's Disease.Biomolecules. 2022 Feb 1;12(2):243. doi: 10.3390/biom12020243. Biomolecules. 2022. PMID: 35204744 Free PMC article. Review.
-
Anti-dyskinetic mechanisms of amantadine and dextromethorphan in the 6-OHDA rat model of Parkinson's disease: role of NMDA vs. 5-HT1A receptors.Eur J Neurosci. 2012 Nov;36(9):3224-34. doi: 10.1111/j.1460-9568.2012.08243.x. Epub 2012 Aug 3. Eur J Neurosci. 2012. PMID: 22861201 Free PMC article.
-
Anatomy of Graft-induced Dyskinesias: Circuit Remodeling in the Parkinsonian Striatum.Basal Ganglia. 2012 Mar 1;2(1):15-30. doi: 10.1016/j.baga.2012.01.002. Epub 2012 Feb 11. Basal Ganglia. 2012. PMID: 22712056 Free PMC article.
-
2016 Philip S. Portoghese Medicinal Chemistry Lectureship: Designing Bivalent or Bitopic Molecules for G-Protein Coupled Receptors. The Whole Is Greater Than the Sum of Its Parts.J Med Chem. 2020 Mar 12;63(5):1779-1797. doi: 10.1021/acs.jmedchem.9b01105. Epub 2019 Sep 24. J Med Chem. 2020. PMID: 31499001 Free PMC article.
References
-
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HHM. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. Journal Neurochemistry. 1995;65:1157–1165. - PubMed
-
- Barik S, de Beaurepaire R. Evidence for a functional role of the dopamine D3 receptors in the cerebellum. Brain Research. 1996;737:347–350. - PubMed
-
- Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskineia: Potential for new therapies. Nature Reviews: Neuroscience. 2001;2:577–588. - PubMed
-
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Research. 1991;564:203–219. - PubMed
-
- Canales JJ, Iversen SD. Psychomotor-activating effects mediated by dopamine D(2) and D(3) receptors in the nucleus accumbens. Pharmacology Biochemistry & Behavior. 2000;67:161–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

