Targeting erbB receptors
- PMID: 20850557
- PMCID: PMC5940346
- DOI: 10.1016/j.semcdb.2010.09.005
Targeting erbB receptors
Abstract
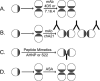
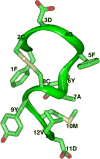
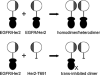
Our work is concerned with the origins and therapy of human cancers. Members of the epidermal growth factor receptor (EGFR) family of tyrosine kinases, also known as erbB or HER receptors, are over expressed and/or activated in many types of human tumors and represent important therapeutic targets in cancer therapy. Studies from our laboratory identified targeted therapy as a way to treat cancer. Rational therapeutics targeting and disabling erbB receptors have been developed to reverse the malignant properties of tumors. Reversal of the malignant phenotype, best seen with disabling the HER2 receptors using monoclonal antibodies is a distinct process from that seen with blocking of ligand binding to cognate receptors as has been done for EGFr receptors. Here we review the mechanisms of action deduced from a number of approaches developed in our laboratory and elsewhere, including monoclonal antibodies, peptide mimetics, recombinant proteins and small molecules. The biochemical and biological principles which have been uncovered during these studies of disabling HER2 homomeric or HER2-EGFr heteromeric receptors will help the development of novel and more efficient therapeutics targeting erbB family receptors.
Copyright © 2010. Published by Elsevier Ltd.
Figures
Similar articles
-
Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review.
-
Targeting ErbB receptors in high-grade glioma.Curr Pharm Des. 2011;17(23):2468-87. doi: 10.2174/138161211797249233. Curr Pharm Des. 2011. PMID: 21827413 Review.
-
ErbB receptors: from oncogenes to targeted cancer therapies.J Clin Invest. 2007 Aug;117(8):2051-8. doi: 10.1172/JCI32278. J Clin Invest. 2007. PMID: 17671639 Free PMC article. Review.
-
Therapeutic targeting of the epidermal growth factor receptor in human cancer.Crit Rev Oncog. 2012;17(1):31-50. doi: 10.1615/critrevoncog.v17.i1.40. Crit Rev Oncog. 2012. PMID: 22471663 Review.
-
Therapeutic monoclonal antibodies for the ErbB family of receptor tyrosine kinases.Cancer Biol Ther. 2003 Jul-Aug;2(4 Suppl 1):S122-6. Cancer Biol Ther. 2003. PMID: 14508089 Review.
Cited by
-
Vaccination targeting human HER3 alters the phenotype of infiltrating T cells and responses to immune checkpoint inhibition.Oncoimmunology. 2017 Apr 12;6(6):e1315495. doi: 10.1080/2162402X.2017.1315495. eCollection 2017. Oncoimmunology. 2017. PMID: 28680745 Free PMC article.
-
An efficient and cost-effective approach to kahalalide F N-terminal modifications using a nuisance algal bloom of Bryopsis pennata.Biochim Biophys Acta. 2015 Sep;1850(9):1849-54. doi: 10.1016/j.bbagen.2015.05.004. Epub 2015 May 9. Biochim Biophys Acta. 2015. PMID: 25964068 Free PMC article.
-
Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer.Cancers (Basel). 2018 Sep 20;10(10):342. doi: 10.3390/cancers10100342. Cancers (Basel). 2018. PMID: 30241301 Free PMC article. Review.
-
Identification of key pathways and transcription factors related to Parkinson disease in genome wide.Mol Biol Rep. 2012 Dec;39(12):10881-7. doi: 10.1007/s11033-012-1985-1. Epub 2012 Oct 18. Mol Biol Rep. 2012. PMID: 23076523
-
Activation of ErbB2 and Downstream Signalling via Rho Kinases and ERK1/2 Contributes to Diabetes-Induced Vascular Dysfunction.PLoS One. 2013 Jun 27;8(6):e67813. doi: 10.1371/journal.pone.0067813. Print 2013. PLoS One. 2013. PMID: 23826343 Free PMC article.
References
-
- Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC, Jr, et al. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25(52):6986–96. - PubMed
-
- De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214(3):559–67. - PubMed
-
- Drebin JA, Link VC, Greene MI. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic antitumor effects in vivo. Oncogene. 1988;2(3):273–7. - PubMed
-
- Zhang H, Wang Q, Montone KT, Peavey JE, Drebin JA, Greene MI, et al. Shared antigenic epitopes and pathobiological functions of anti-p185(her2/neu) monoclonal antibodies. Oncogene. 1999;67(1):15–25. - PubMed
-
- Hu S, Zhu Z, Li L, Chang L, Li W, Cheng L, et al. Epitope mapping and structural analysis of an anti-ErbB2 antibody A21: molecular basis for tumor inhibitory mechanism. Proteins. 2008;70(3):938–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

