Internalizing cancer antibodies from phage libraries selected on tumor cells and yeast-displayed tumor antigens
- PMID: 20851130
- PMCID: PMC4195448
- DOI: 10.1016/j.jmb.2010.09.006
Internalizing cancer antibodies from phage libraries selected on tumor cells and yeast-displayed tumor antigens
Abstract
A number of approaches have been utilized to generate antibodies to cancer cell surface receptors that can be used as potential therapeutics. A number of these therapeutic approaches, including antibody-drug conjugates, immunotoxins, and targeted nucleic acid delivery, require antibodies that not only bind receptor but also undergo internalization into the cell upon binding. We previously reported on the ability to generate cancer cell binding and internalizing antibodies directly from human phage antibody libraries selected for internalization into cancer cell lines. While a number of useful antibodies have been generated using this approach, limitations include the inability to direct the selections to specific antigens and to identify the antigen bound by the antibodies. Here we show that these limitations can be overcome by using yeast-displayed antigens known to be associated with a cell type to select the phage antibody output after several rounds of selection on a mammalian cell line. We used this approach to generate several human phage antibodies to yeast-displayed EphA2 and CD44. The antibodies bound both yeast-displayed and mammalian cell surface antigens, and were endocytosed upon binding to mammalian cells. This approach is generalizable to many mammalian cell surface proteins, results in the generation of functional internalizing antibodies, and does not require antigen expression and purification for antibody generation.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
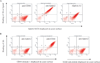


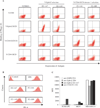
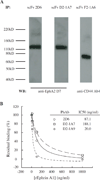

References
-
- Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. - PubMed
-
- Hainsworth JD, Burris HA, 3rd, Morrissey LH, Litchy S, Scullin DC, Jr, Bearden JD, 3rd, Richards P, Greco FA. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma. Blood. 2000;95:3052–3056. - PubMed
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. - PubMed
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
-
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

