5-Lipoxygenase in mouse cerebellar Purkinje cells
- PMID: 20851170
- PMCID: PMC2975899
- DOI: 10.1016/j.neuroscience.2010.09.019
5-Lipoxygenase in mouse cerebellar Purkinje cells
Abstract
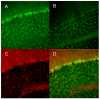
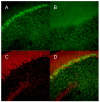
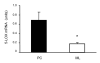
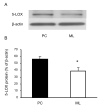
It has been suggested that the enzymatic pathway of 5-lipoxygenase (5-LOX) influences brain functioning and pathobiology. The mRNAs for both the enzyme 5-LOX and its activating protein FLAP have been found in the cerebellum. In this work, we investigated the cellular expression of 5-LOX in the adult mouse cerebellar cortex. We used the in situ mRNA hybridization assay, immunocytochemistry, laser capture microdissection, and our previously developed method for assaying the DNA methylation status of a putative mouse 5-LOX promoter. Since both 5-LOX mRNA in situ hybridization signal and FLAP immunoreactivity co-localize with calbindin 28 kD immunoreactivity (a Purkinje cell marker) but not with S-100β immunoreactivity (a Bergmann glia marker), the suggestion is that the 5-LOX pathway is expressed in cerebellar Purkinje cells. We found that methylation in the sites targeted by methylation-sensitive restriction endonucleases AciI and HinP1I but not BstUI and HpaII was greater in DNA samples obtained from a high-5-LOX-expressing cerebellar region (Purkinje cells) versus a low-5-LOX-expressing region (the molecular cell layer), suggesting a possible epigenetic contribution to the cell-specific 5-LOX expression in the cerebellum. We propose that Purkinje cell-localized 5-LOX and FLAP expression may be involved in the cerebellar synthesis of leukotrienes and/or could influence the Dicer-mediated microRNA formation and processes of neuroplasticity.
Copyright © 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
5-Lipoxygenase and epigenetic DNA methylation in aging cultures of cerebellar granule cells.Neuroscience. 2009 Dec 29;164(4):1531-7. doi: 10.1016/j.neuroscience.2009.09.039. Epub 2009 Sep 22. Neuroscience. 2009. PMID: 19778587 Free PMC article.
-
5-Lipoxygenase DNA methylation and mRNA content in the brain and heart of young and old mice.Neural Plast. 2009;2009:209596. doi: 10.1155/2009/209596. Epub 2009 Dec 13. Neural Plast. 2009. PMID: 20052386 Free PMC article.
-
Inflammatory 5-LOX mRNA and protein are increased in brain of aging rats.Neurobiol Aging. 2000 Sep-Oct;21(5):647-52. doi: 10.1016/s0197-4580(00)00167-6. Neurobiol Aging. 2000. PMID: 11016533
-
Mode of action of the leukotriene synthesis (FLAP) inhibitor BAY X 1005: implications for biological regulation of 5-lipoxygenase.Agents Actions. 1994 Nov;43(1-2):64-8. doi: 10.1007/BF02005767. Agents Actions. 1994. PMID: 7741044 Review.
-
Putative role of neuronal 5-lipoxygenase in an aging brain.FASEB J. 2000 Jul;14(10):1464-9. doi: 10.1096/fj.14.10.1464. FASEB J. 2000. PMID: 10877840 Review.
Cited by
-
Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids.NPJ Aging Mech Dis. 2016 Jun 23;2:16012. doi: 10.1038/npjamd.2016.12. eCollection 2016. NPJ Aging Mech Dis. 2016. PMID: 28721267 Free PMC article.
-
Effect of aging on 5-hydroxymethylcytosine in the mouse hippocampus.Restor Neurol Neurosci. 2012;30(3):237-45. doi: 10.3233/RNN-2012-110223. Restor Neurol Neurosci. 2012. PMID: 22426040 Free PMC article.
-
Effect of aging on 5-hydroxymethylcytosine in brain mitochondria.Neurobiol Aging. 2012 Dec;33(12):2881-91. doi: 10.1016/j.neurobiolaging.2012.02.006. Epub 2012 Mar 22. Neurobiol Aging. 2012. PMID: 22445327 Free PMC article.
References
-
- Bijlsma MF, Peppelenbosch MP, Spek CA, Roelink H. Leukotriene synthesis is required for hedgehog-dependent neurite projection in neuralized embryoid bodies but not for motor neuron differentiation. Stem Cells. 2008;26:1138–1145. - PubMed
-
- Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. - PubMed
-
- Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007;28:1457–1462. - PubMed
-
- Cristino L, Starowicz K, De Petrocellis L, Morishita J, Ueda N, Guglielmotti V, Di Marzo V. Immunohistochemical localization of anabolic and catabolic enzymes for anandamide and other putative endovanilloids in the hippocampus and cerebellar cortex of the mouse brain. Neuroscience. 2008;151:955–968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

