The quaternary organization and dynamics of the molecular chaperone HSP26 are thermally regulated
- PMID: 20851350
- PMCID: PMC3388541
- DOI: 10.1016/j.chembiol.2010.06.016
The quaternary organization and dynamics of the molecular chaperone HSP26 are thermally regulated
Abstract
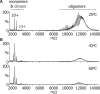
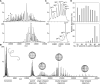
The function of ScHSP26 is thermally controlled: the heat shock that causes the destabilization of target proteins leads to its activation as a molecular chaperone. We investigate the structural and dynamical properties of ScHSP26 oligomers through a combination of multiangle light scattering, fluorescence spectroscopy, NMR spectroscopy, and mass spectrometry. We show that ScHSP26 exists as a heterogeneous oligomeric ensemble at room temperature. At heat-shock temperatures, two shifts in equilibria are observed: toward dissociation and to larger oligomers. We examine the quaternary dynamics of these oligomers by investigating the rate of exchange of subunits between them and find that this not only increases with temperature but proceeds via two separate processes. This is consistent with a conformational change of the oligomers at elevated temperatures which regulates the disassembly rates of this thermally activated protein.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures



References
-
- Aquilina JA, Benesch JLP, Ding LL, Yaron O, Horwitz J, Robinson CV. Subunit exchange of polydisperse proteins: mass spectrometry reveals consequences of alphaA-crystallin truncation. J. Biol. Chem. 2005;280:14485–14491. - PubMed
-
- Basha E, Lee GJ, Demeler B, Vierling E. Chaperone activity of cytosolic small heat shock proteins from wheat. Eur. J. Biochem. 2004;271:1426–1436. - PubMed
-
- Benesch JLP. Collisional activation of protein complexes: picking up the pieces. J. Am. Soc. Mass Spectrom. 2009;20:341–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

