PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial
- PMID: 20851460
- PMCID: PMC2956883
- DOI: 10.1016/S0140-6736(10)61086-0
PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial
Abstract
Background: Innovative prevention strategies for HIV-1 transmission are urgently needed. PRO2000 vaginal gel was efficacious against HIV-1 transmission in studies in macaques; we aimed to assess efficacy and safety of 2% and 0·5% PRO2000 gels against vaginal HIV-1 transmission in women in sub-Saharan Africa.
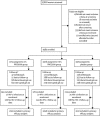
Methods: Microbicides Development Programme 301 was a phase 3, randomised, double-blind, parallel-group trial, undertaken at 13 clinics in South Africa, Tanzania, Uganda, and Zambia. We randomly assigned sexually active women, aged 18 years or older (≥16 years in Tanzania and Uganda) without HIV-1 infection in a 1:1:1 ratio to 2% PRO2000, 0·5% PRO2000, or placebo gel groups for 52 weeks (up to 104 weeks in Uganda). Randomisation was done by computerised random number generator. Investigators and participants were masked to group assignment. The primary efficacy outcome was incidence of HIV-1 infection before week 52, which was censored for pregnancy and excluded participants without HIV-1 follow-up data or with HIV-1 infection at enrolment. HIV-1 status was established by rapid tests or ELISA at screening at 12 weeks, 24 weeks, 40 weeks, and 52 weeks, and confirmed in a central reference laboratory. The primary safety endpoint was an adverse event of grade 3 or worse. Use of 2% PRO2000 gel was discontinued on Feb 14, 2008, on the recommendation of the Independent Data Monitoring Committee because of low probability of benefit. This trial is registered at http://isrctn.org, number ISRCTN 64716212.
Findings: We enrolled 9385 of 15 818 women screened. 2591 (95%) of 2734 participants enrolled to the 2% PRO2000 group, 3156 (95%) of 3326 in the 0·5% PRO2000 group, and 3112 (94%) of 3325 in the placebo group were included in the primary efficacy analysis. Mean reported gel use at last sex act was 89% (95% CI 86-91). HIV-1 incidence was much the same between groups at study end (incidence per 100 woman-years was 4·5 [95% CI 3·8-5·4] for 0·5% PRO2000 vs 4·3 [3·6-5·2] for placebo, hazard ratio 1·05 [0·82-1·34], p=0·71), and at discontinuation (4·7 [3·8-5·8] for 2% PRO2000 gel, 3·9 [3·0-4·9] for 0·5% PRO2000 gel, and 3·9 [3·1-5·0] for placebo gel). Incidence of the primary safety endpoint at study end was 4·6 per 100 woman-years (95% CI 3·9-5·4) in the 0·5% PRO2000 group and 3·9 (3·2-4·6) in the placebo group; and was 4·5 (3·7-5·5) in the 2% PRO2000 group at discontinuation.
Interpretation: Although safe, 0·5% PRO2000 and 2% PRO2000 are not efficacious against vaginal HIV-1 transmission and are not indicated for this use.
Funding: UK Department for International Development, UK Medical Research Council, European and Developing Countries Clinical Trials Partnership, International Partnership for Microbicides, and Endo Pharmaceuticals Solutions.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Preventing HIV infection: turning the tide for young women.Lancet. 2010 Oct 16;376(9749):1281-2. doi: 10.1016/S0140-6736(10)61309-8. Epub 2010 Sep 17. Lancet. 2010. PMID: 20851461 No abstract available.
References
-
- UNAIDS/WHO AIDS epidemic update, Joint United Nations Programme on HIV/AIDS (UNAIDS) 2009. http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf (accessed Aug 11, 2010). - PubMed
-
- Skoler-Karpoff S, Ramjee G, Ahmed K. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. - PubMed
-
- Van Damme L, Govinden R, Mirembe FM. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–472. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

