Complement mediated hepatocytes injury in a model of autoantibody induced hepatitis
- PMID: 20851495
- PMCID: PMC3557916
- DOI: 10.1016/j.imbio.2010.08.004
Complement mediated hepatocytes injury in a model of autoantibody induced hepatitis
Abstract
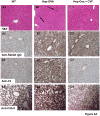
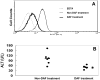
Despite multiple reports on autoantibody-initiated complement activation in autoimmune hepatitis (AIH), how does the humoral immunity contribute to the pathogenesis of AIH remained unclear. In this report, by adoptively transferring a polyclonal rabbit anti-OVA antibody into Hep-OVA Tg mice in which OVA is selectively expressed on the surface of hepatocytes, we found that excessive complement activation initiated by the autoantibody overwhelmed the protection of intrinsic cell surface complement regulators, and induced hepatocytes injury both in vitro and in vivo. The anti-OVA antibody induced hepatic injury in Hep-OVA Tg but not WT C57BL/6 mice as assessed by serum ALT levels and liver histopathology. Immunohistochemical analyses showed that after the antibody administration, there was massive complement activation on anti-OVA IgG coated hepatocytes in Hep-OVA Tg mice, but not in WT mice. Consistent with these results, depleting complement by cobra venom factor (CVF) prior to antibody injections protected Hep-OVA Tg mice from anti-OVA IgG induced hepatic injury. In addition, treating Hep-OVA Tg mice with recombinant mouse decay accelerating factor, a native complement inhibitor, protected them from autoantibody induced hepatitis. These results suggest that complement could play a pivotal role in liver specific autoantibody mediated hepatocyte injury in AIH, and that complement inhibitors could be, in principle, developed as novel therapeutics against AIH.
Copyright © 2010 Elsevier GmbH. All rights reserved.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures
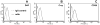




Similar articles
-
A murine model of acute liver injury induced by human monoclonal autoantibody.Hepatology. 2005 Jul;42(1):149-55. doi: 10.1002/hep.20726. Hepatology. 2005. PMID: 15918155
-
Hepatitis resulting from liver-specific expression and recognition of self-antigen.J Autoimmun. 2008 Nov;31(3):208-15. doi: 10.1016/j.jaut.2008.04.015. Epub 2008 Jun 2. J Autoimmun. 2008. PMID: 18513923 Free PMC article.
-
Humoral immune mechanism of liver injury in giant cell hepatitis with autoimmune hemolytic anemia.J Pediatr Gastroenterol Nutr. 2014 Jan;58(1):74-80. doi: 10.1097/MPG.0b013e3182a98dbe. J Pediatr Gastroenterol Nutr. 2014. PMID: 23969541
-
Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases.Int Immunopharmacol. 2001 Mar;1(3):445-59. doi: 10.1016/s1567-5769(00)00043-6. Int Immunopharmacol. 2001. PMID: 11367529 Review.
-
The role of complement activation in autoimmune liver disease.Autoimmun Rev. 2020 Jun;19(6):102534. doi: 10.1016/j.autrev.2020.102534. Epub 2020 Mar 28. Autoimmun Rev. 2020. PMID: 32234403 Review.
Cited by
-
Complementary serum proteomic analysis of autoimmune hepatitis in mice and patients.J Transl Med. 2013 Jun 13;11:146. doi: 10.1186/1479-5876-11-146. J Transl Med. 2013. PMID: 23763817 Free PMC article.
-
Toward a new autoantibody diagnostic orthodoxy: understanding the bad, good and indifferent.Auto Immun Highlights. 2012 Mar 21;3(2):51-8. doi: 10.1007/s13317-012-0030-7. eCollection 2012 Aug. Auto Immun Highlights. 2012. PMID: 26000127 Free PMC article. Review.
-
Mesenchymal stem cells are injured by complement after their contact with serum.Blood. 2012 Oct 25;120(17):3436-43. doi: 10.1182/blood-2012-03-420612. Epub 2012 Sep 10. Blood. 2012. PMID: 22966167 Free PMC article.
-
Expression of HLA and Autoimmune Pathway Genes in Liver Biopsies of Young Subjects With Autoimmune Hepatitis Type 1.J Pediatr Gastroenterol Nutr. 2022 Sep 1;75(3):269-275. doi: 10.1097/MPG.0000000000003538. Epub 2022 Jun 27. J Pediatr Gastroenterol Nutr. 2022. PMID: 35759748 Free PMC article.
-
Autoimmune hepatitis as a unique form of an autoimmune liver disease: immunological aspects and clinical overview.Autoimmune Dis. 2012;2012:312817. doi: 10.1155/2012/312817. Epub 2012 Dec 12. Autoimmune Dis. 2012. PMID: 23304455 Free PMC article.
References
-
- Czaja AJ. Autoimmune liver disease. Curr Opin Gastroenterol. 2008;24:298. - PubMed
-
- Czaja AJ. Autoimmune liver disease. Curr Opin Gastroenterol. 2009;25:215. - PubMed
-
- Treichel U, McFarlane BM, Seki T, Krawitt EL, Alessi N, Stickel F, McFarlane IG, Kiyosawa K, Furuta S, Freni MA, et al. Demographics of anti-asialoglycoprotein receptor autoantibodies in autoimmune hepatitis. Gastroenterology. 1994;107:799. - PubMed
-
- Yamauchi K, Yamaguchi N, Furukawa T, Takatsu K, Nakanishi T, Sasaki M, Isono E, Tokushige K, Komatsu T, Shiratori K. A novel IgM class autoantibody to a hepatocyte-related 190 kDa molecule in patients with type 1 autoimmune hepatitis. Hepatology. 2004;40:687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

