Frequency control of cell cycle oscillators
- PMID: 20851595
- PMCID: PMC3522487
- DOI: 10.1016/j.gde.2010.08.006
Frequency control of cell cycle oscillators
Abstract
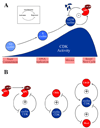
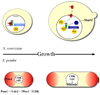
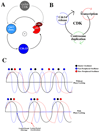
The cell cycle oscillator, based on a core negative feedback loop and modified extensively by positive feedback, cycles with a frequency that is regulated by environmental and developmental programs to encompass a wide range of cell cycle times. We discuss how positive feedback allows frequency tuning, how size and morphogenetic checkpoints regulate oscillator frequency, and how extrinsic oscillators such as the circadian clock gate cell cycle frequency. The master cell cycle regulatory oscillator in turn controls the frequency of peripheral oscillators controlling essential events. A recently proposed phase-locking model accounts for this coupling.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Circadian clocks and cell division: what's the pacemaker?Cell Cycle. 2010 Oct 1;9(19):3864-73. doi: 10.4161/cc.9.19.13205. Epub 2010 Oct 1. Cell Cycle. 2010. PMID: 20890114 Free PMC article.
-
Contribution of membrane-associated oscillators to biological timing at different timescales.Front Physiol. 2024 Jan 9;14:1243455. doi: 10.3389/fphys.2023.1243455. eCollection 2023. Front Physiol. 2024. PMID: 38264332 Free PMC article.
-
A detailed map of coupled circadian clock and cell cycle with qualitative dynamics validation.BMC Bioinformatics. 2021 May 11;22(1):240. doi: 10.1186/s12859-021-04158-9. BMC Bioinformatics. 2021. PMID: 33975535 Free PMC article.
-
Regulation of tissue regeneration by the circadian clock.Eur J Neurosci. 2021 Jun;53(11):3576-3597. doi: 10.1111/ejn.15244. Epub 2021 May 14. Eur J Neurosci. 2021. PMID: 33893679 Review.
-
Biological timing and the clock metaphor: oscillatory and hourglass mechanisms.Chronobiol Int. 2001 May;18(3):329-69. doi: 10.1081/cbi-100103961. Chronobiol Int. 2001. PMID: 11475408 Review.
Cited by
-
The Inner Nuclear Membrane Protein Src1 Is Required for Stable Post-Mitotic Progression into G1 in Aspergillus nidulans.PLoS One. 2015 Jul 6;10(7):e0132489. doi: 10.1371/journal.pone.0132489. eCollection 2015. PLoS One. 2015. PMID: 26147902 Free PMC article.
-
Roles for the Histone Modifying and Exchange Complex NuA4 in Cell Cycle Progression in Drosophila melanogaster.Genetics. 2016 Jul;203(3):1265-81. doi: 10.1534/genetics.116.188581. Epub 2016 May 16. Genetics. 2016. PMID: 27184390 Free PMC article.
-
Oscillation of clock and clock controlled genes induced by serum shock in human breast epithelial and breast cancer cells: regulation by melatonin.Breast Cancer (Auckl). 2012;6:137-50. doi: 10.4137/BCBCR.S9673. Epub 2012 Sep 13. Breast Cancer (Auckl). 2012. PMID: 23012497 Free PMC article.
-
Hippo signaling is intrinsically regulated during cell cycle progression by APC/CCdh1.Proc Natl Acad Sci U S A. 2019 May 7;116(19):9423-9432. doi: 10.1073/pnas.1821370116. Epub 2019 Apr 18. Proc Natl Acad Sci U S A. 2019. PMID: 31000600 Free PMC article.
-
Design of Oscillatory Networks through Post-Translational Control of Network Components.Synth Biol Eng. 2023 Jun;1(1):10004. doi: 10.35534/sbe.2023.10004. Epub 2023 Mar 13. Synth Biol Eng. 2023. PMID: 38590452 Free PMC article.
References
-
- Tyson JJ, Chen KC, Novak B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol. 2003;15:221–231. - PubMed
-
- Felix MA, Labbe JC, Doree M, Hunt T, Karsenti E. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature. 1990;346:379–382. - PubMed
-
- Dunlap JC, Loros JJ, Colot HV, Mehra A, Belden WJ, Shi M, Hong CI, Larrondo LF, Baker CL, Chen CH, et al. A circadian clock in Neurospora: how genes and proteins cooperate to produce a sustained, entrainable, and compensated biological oscillator with a period of about a day. Cold Spring Harb Symp Quant Biol. 2007;72:57–68. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

