Solid-state NMR paramagnetic relaxation enhancement immersion depth studies in phospholipid bilayers
- PMID: 20851650
- PMCID: PMC2978330
- DOI: 10.1016/j.jmr.2010.08.012
Solid-state NMR paramagnetic relaxation enhancement immersion depth studies in phospholipid bilayers
Abstract
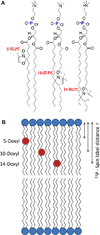
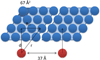
A new approach for determining the membrane immersion depth of a spin-labeled probe has been developed using paramagnetic relaxation enhancement (PRE) in solid-state NMR spectroscopy. A DOXYL spin label was placed at different sites of 1-palmitoyl-2-stearoyl-sn-glycero-3-phosphocholine (PSPC) phospholipid bilayers as paramagnetic moieties and the resulting enhancements of the longitudinal relaxation (T₁) times of ³¹P nuclei on the surface of the bilayers were measured by a standard inversion recovery pulse sequence. The ³¹P NMR spin-lattice relaxation times decrease steadily as the DOXYL spin label moves closer to the surface as well as the concentration of the spin-labeled lipids increase. The enhanced relaxation vs. the position and concentration of spin-labels indicate that PRE induced by the DOXYL spin label are significant to determine longer distances over the whole range of the membrane depths. When these data were combined with estimated correlation times τ(c), the r⁻⁶-weighted, time-averaged distances between the spin-labels and the ³¹P nuclei on the membrane surface were estimated. The application of using this solid-state NMR PRE approach coupled with site-directed spin labeling (SDSL) may be a powerful method for measuring membrane protein immersion depth.
Published by Elsevier Inc.
Figures



Similar articles
-
Solid-state NMR (31)P paramagnetic relaxation enhancement membrane protein immersion depth measurements.J Phys Chem B. 2014 Apr 24;118(16):4370-7. doi: 10.1021/jp500267y. Epub 2014 Apr 11. J Phys Chem B. 2014. PMID: 24689497 Free PMC article.
-
The distribution of lipid attached spin probes in bilayers: application to membrane protein topology.Biophys J. 2003 Sep;85(3):1691-701. doi: 10.1016/S0006-3495(03)74599-8. Biophys J. 2003. PMID: 12944284 Free PMC article.
-
Molecular dynamics simulations of depth distribution of spin-labeled phospholipids within lipid bilayer.J Phys Chem B. 2013 May 16;117(19):5875-85. doi: 10.1021/jp4026706. Epub 2013 May 8. J Phys Chem B. 2013. PMID: 23614631 Free PMC article.
-
The measurement of immersion depth and topology of membrane proteins by solution state NMR.Biochim Biophys Acta. 2007 Dec;1768(12):3044-51. doi: 10.1016/j.bbamem.2007.09.011. Epub 2007 Oct 16. Biochim Biophys Acta. 2007. PMID: 17976526 Review.
-
Time-resolved electron spin resonance studies of spin-labelled lipids in membranes.Chem Phys Lipids. 2006 Jun;141(1-2):142-57. doi: 10.1016/j.chemphyslip.2006.02.009. Epub 2006 Mar 13. Chem Phys Lipids. 2006. PMID: 16564516 Review.
Cited by
-
Measuring membrane penetration with depth-dependent fluorescence quenching: distribution analysis is coming of age.Biochim Biophys Acta. 2014 Sep;1838(9):2289-95. doi: 10.1016/j.bbamem.2014.02.019. Epub 2014 Mar 1. Biochim Biophys Acta. 2014. PMID: 24593994 Free PMC article. Review.
-
Peridinin Is an Exceptionally Potent and Membrane-Embedded Inhibitor of Bilayer Lipid Peroxidation.J Am Chem Soc. 2018 Nov 14;140(45):15227-15240. doi: 10.1021/jacs.8b06933. Epub 2018 Nov 2. J Am Chem Soc. 2018. PMID: 30388000 Free PMC article.
-
Solid-state NMR (31)P paramagnetic relaxation enhancement membrane protein immersion depth measurements.J Phys Chem B. 2014 Apr 24;118(16):4370-7. doi: 10.1021/jp500267y. Epub 2014 Apr 11. J Phys Chem B. 2014. PMID: 24689497 Free PMC article.
-
Extraction, Characterization, and Anticoagulant Activity of a Sulfated Polysaccharide from Bursatella leachii Viscera.ACS Omega. 2020 Jun 10;5(24):14786-14795. doi: 10.1021/acsomega.0c01724. eCollection 2020 Jun 23. ACS Omega. 2020. PMID: 32596616 Free PMC article.
-
Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop.J Extracell Vesicles. 2019 Nov 8;8(1):1684862. doi: 10.1080/20013078.2019.1684862. eCollection 2019. J Extracell Vesicles. 2019. PMID: 31762963 Free PMC article.
References
-
- Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta-Biomembr. 1999;1462:55–70. - PubMed
-
- Popot JL, Engelman DM. Helical membrane protein folding, stability, and evolution. Annu. Rev. Biochem. 2000;69:881–922. - PubMed
-
- Hong M. Structure, topology, and dynamics of membrane peptides and proteins from solid-state NMR Spectroscopy. J. Phys. Chem. B. 2007;111:10340–10351. - PubMed
-
- Bechinger B. Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Curr. Opin. Colloid Interface Sci. 2009;14:349–355.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

