Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study
- PMID: 20851682
- PMCID: PMC3107731
- DOI: 10.1016/S1470-2045(10)70203-5
Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study
Abstract
Background: Chemotherapy has historically proven ineffective in advanced differentiated thyroid cancers, but the realisation that various tyrosine kinases are activated in the disease suggested a potential therapeutic role for tyrosine-kinase inhibitors. We investigated the safety and efficacy of pazopanib.
Methods: This phase 2 trial was done from Feb 22, 2008, to Jan 31, 2009, in patients with metastatic, rapidly progressive, radioiodine-refractory differentiated thyroid cancers. Each patient received 800 mg continuous pazopanib daily in 4-week cycles until disease progression, drug intolerance, or both occurred. Up to two previous therapies were allowed, and measurable disease with radiographic progression in the 6-month period before enrolment was a requirement for inclusion. The primary endpoint was any tumour response, according to the Response Evaluation Criteria in Solid Tumors 1.0. This study is registered with ClinicalTrials.gov, number NCT00625846.
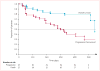
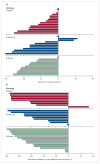
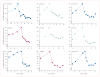
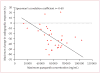
Findings: 39 patients were enrolled. One patient had received no previous radioiodine therapy and another withdrew consent before treatment. Clinical outcomes could, therefore, be assessed in 37 patients (19 [51%] men, median age 63 years). The study is closed to accrual of new patients, but several enrolled patients are still being treated. Patients received a median of 12 cycles (range 1 to >23, total >383). Confirmed partial responses were recorded in 18 patients (response rate 49%, 95% CI 35-68), with likelihood of response lasting longer than 1 year calculated to be 66%. Maximum concentration of pazopanib in plasma during cycle one was significantly correlated with radiographic response (r=-0·40, p=0·021). 16 (43%) patients required dose reductions owing to adverse events, the most frequent of which (any grade) were fatigue (29 patients), skin and hair hypopigmentation (28), diarrhoea (27), and nausea (27). Two patients who died during treatment had pre-existing contributory disorders.
Interpretation: Pazopanib seems to represent a promising therapeutic option for patients with advanced differentiated thyroid cancers. The correlation of the patient's response and pazopanib concentration during the first cycle might indicate that treatment can be individualised to achieve optimum outcomes. Assessment of pazopanib in an expanded cohort of patients with differentiated thyroid cancer, as well as in cohorts of patients with medullary and anaplastic thyroid cancers, is presently being done.
Funding: National Cancer Institute, supported in part by NCI CA15083 and CM62205.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures
Comment in
-
Kinase inhibitors for refractory thyroid cancers.Lancet Oncol. 2010 Oct;11(10):912-3. doi: 10.1016/S1470-2045(10)70226-6. Epub 2010 Sep 17. Lancet Oncol. 2010. PMID: 20851683 No abstract available.
References
-
- American Cancer Society. Cancer facts & figures 2009. Atlanta: American Cancer Society; 2009.
-
- Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–07. - PubMed
-
- Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) program. Oncologist. 2007;12:20–37. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

