Targeted nanoagents for the detection of cancers
- PMID: 20851695
- PMCID: PMC2981649
- DOI: 10.1016/j.molonc.2010.08.003
Targeted nanoagents for the detection of cancers
Abstract
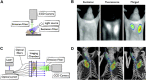
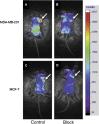
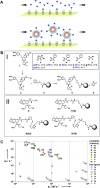
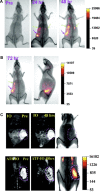
Nanotechnology has enabled a renaissance in the diagnosis of cancers. This is due, in part to the ability to develop agents bearing multiple functionalities, including those utilized for targeting, imaging, and therapy, allowing for the tailoring of the properties of the nanomaterials. Whereas many nanomaterials exhibit localization to diseased tissues via intrinsic targeting, the addition of targeting ligands, such as antibodies, peptides, aptamers, and small molecules, facilitates far more sensitive cancer detection. As such, this review focuses upon some of the most poignant examples of the utility of affinity ligand targeted nanoagents in the detection of cancer.
Copyright © 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Applications of aptamers in targeted imaging: state of the art.Curr Top Med Chem. 2015;15(12):1138-52. doi: 10.2174/1568026615666150413153400. Curr Top Med Chem. 2015. PMID: 25866268 Free PMC article. Review.
-
Aptamers: active targeting ligands for cancer diagnosis and therapy.Theranostics. 2015 Jan 20;5(4):322-44. doi: 10.7150/thno.10257. eCollection 2015. Theranostics. 2015. PMID: 25699094 Free PMC article. Review.
-
Aptamers in targeted nanotherapy.Curr Top Med Chem. 2015;15(12):1102-14. doi: 10.2174/1568026615666150413153525. Curr Top Med Chem. 2015. PMID: 25866269 Review.
-
Functional DNA-containing nanomaterials: cellular applications in biosensing, imaging, and targeted therapy.Acc Chem Res. 2014 Jun 17;47(6):1891-901. doi: 10.1021/ar500078f. Epub 2014 Apr 29. Acc Chem Res. 2014. PMID: 24780000 Free PMC article.
-
Supramolecular peptide nano-assemblies for cancer diagnosis and therapy: from molecular design to material synthesis and function-specific applications.J Nanobiotechnology. 2021 Aug 23;19(1):253. doi: 10.1186/s12951-021-00999-x. J Nanobiotechnology. 2021. PMID: 34425823 Free PMC article. Review.
Cited by
-
Emerging role of radiolabeled nanoparticles as an effective diagnostic technique.EJNMMI Res. 2012 Jul 18;2(1):39. doi: 10.1186/2191-219X-2-39. EJNMMI Res. 2012. PMID: 22809406 Free PMC article.
-
NANOMEDICINE: will it offer possibilities to overcome multiple drug resistance in cancer?J Nanobiotechnology. 2016 Mar 9;14:17. doi: 10.1186/s12951-016-0172-2. J Nanobiotechnology. 2016. PMID: 26955956 Free PMC article. Review.
-
Tissue proteomics of the human mammary gland: towards an abridged definition of the molecular phenotypes underlying epithelial normalcy.Mol Oncol. 2010 Dec;4(6):539-61. doi: 10.1016/j.molonc.2010.09.005. Epub 2010 Oct 8. Mol Oncol. 2010. PMID: 21036680 Free PMC article.
-
MRI of breast tumor initiating cells using the extra domain-B of fibronectin targeting nanoparticles.Theranostics. 2014 Jun 10;4(8):845-57. doi: 10.7150/thno.8343. eCollection 2014. Theranostics. 2014. PMID: 24955145 Free PMC article.
-
Targeted Magnetic Nanotheranostics of Cancer.Molecules. 2017 Jun 12;22(6):975. doi: 10.3390/molecules22060975. Molecules. 2017. PMID: 28604617 Free PMC article. Review.
References
-
- Aime, S. , Castelli, D.D. , Crich, S.G. , Gianolio, E. , Terreno, E. , 2009. Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications. Acc. Chem. Res. 42, 822–831. - PubMed
-
- American Cancer Society, 2009. Cancer Facts & Figures 2009 American Cancer Society; Atlanta:
-
- Bagalkot, V. , Zhang, L. , Levy-Nissenbaum, E. , Jon, S. , Kantoff, P.W. , Langer, R. , Farokhzad, O.C. , 2007. Quantum dot–aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano. Lett. 7, 3065–3070. - PubMed
-
- Biju, V. , Mundayoor, S. , Omkumar, R.V. , Anas, A. , Ishikawa, M. , 2010. Bioconjugated quantum dots for cancer research: present status, prospects and remaining issues. Biotechnol. Adv. 28, 199–213. - PubMed
-
- Cairns, R.A. , Khokha, R. , Hill, R.P. , 2003. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr. Mol. Med. 3, 659–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

