The farnesoid X receptor regulates adipocyte differentiation and function by promoting peroxisome proliferator-activated receptor-gamma and interfering with the Wnt/beta-catenin pathways
- PMID: 20851881
- PMCID: PMC2978604
- DOI: 10.1074/jbc.M110.166231
The farnesoid X receptor regulates adipocyte differentiation and function by promoting peroxisome proliferator-activated receptor-gamma and interfering with the Wnt/beta-catenin pathways
Abstract
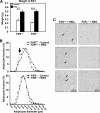
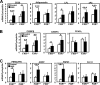
The bile acid receptor farnesoid X receptor (FXR) is expressed in adipose tissue, but its function remains poorly defined. Peroxisome proliferator-activated receptor-γ (PPARγ) is a master regulator of adipocyte differentiation and function. The aim of this study was to analyze the role of FXR in adipocyte function and to assess whether it modulates PPARγ action. Therefore, we tested the responsiveness of FXR-deficient mice (FXR(-/-)) and cells to the PPARγ activator rosiglitazone. Our results show that genetically obese FXR(-/-)/ob/ob mice displayed a resistance to rosiglitazone treatment. In vitro, rosiglitazone treatment did not induce normal adipocyte differentiation and lipid droplet formation in FXR(-/-) mouse embryonic fibroblasts (MEFs) and preadipocytes. Moreover, FXR(-/-) MEFs displayed both an increased lipolysis and a decreased de novo lipogenesis, resulting in reduced intracellular triglyceride content, even upon PPARγ activation. Retroviral-mediated FXR re-expression in FXR(-/-) MEFs restored the induction of adipogenic marker genes during rosiglitazone-forced adipocyte differentiation. The expression of Wnt/β-catenin pathway and target genes was increased in FXR(-/-) adipose tissue and MEFs. Moreover, the expression of several endogenous inhibitors of this pathway was decreased early during the adipocyte differentiation of FXR(-/-) MEFs. These findings demonstrate that FXR regulates adipocyte differentiation and function by regulating two counteracting pathways of adipocyte differentiation, the PPARγ and Wnt/β-catenin pathways.
Figures






Similar articles
-
Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferator-activated receptor-gamma agonists.Endocrinology. 2002 Jun;143(6):2106-18. doi: 10.1210/endo.143.6.8842. Endocrinology. 2002. PMID: 12021175
-
Activation of PPARγ at an Early Stage of Differentiation Enhances Adipocyte Differentiation of MEFs Derived from Type II Diabetic TSOD Mice and Alters Lipid Droplet Morphology.Biol Pharm Bull. 2017;40(6):852-859. doi: 10.1248/bpb.b17-00030. Biol Pharm Bull. 2017. PMID: 28566629
-
The role of prolyl hydroxylase domain protein (PHD) during rosiglitazone-induced adipocyte differentiation.J Biol Chem. 2014 Jan 31;289(5):2755-64. doi: 10.1074/jbc.M113.493650. Epub 2013 Dec 12. J Biol Chem. 2014. PMID: 24338020 Free PMC article.
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
-
Peroxisome proliferator-activated receptor gamma in white and brown adipocyte regulation and differentiation.Physiol Res. 2020 Nov 16;69(5):759-773. doi: 10.33549/physiolres.934411. Epub 2020 Sep 9. Physiol Res. 2020. PMID: 32901494 Free PMC article. Review.
Cited by
-
[Impact of lithocholic acid on the osteogenic and adipogenic differentiation balance of bone marrow mesenchymal stem cells].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024 Jan 15;38(1):82-90. doi: 10.7507/1002-1892.202308050. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024. PMID: 38225846 Free PMC article. Chinese.
-
Regulation of Matrix Remodeling by Peroxisome Proliferator-Activated Receptor-γ: A Novel Link Between Metabolism and Fibrogenesis.Open Rheumatol J. 2012;6:103-15. doi: 10.2174/1874312901206010103. Epub 2012 Jun 15. Open Rheumatol J. 2012. PMID: 22802908 Free PMC article.
-
Farnesoid X Receptor Activation in Brain Alters Brown Adipose Tissue Function via the Sympathetic System.Front Mol Neurosci. 2022 Jan 4;14:808603. doi: 10.3389/fnmol.2021.808603. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35058750 Free PMC article.
-
Myeloma cells shift osteoblastogenesis to adipogenesis by inhibiting the ubiquitin ligase MURF1 in mesenchymal stem cells.Sci Signal. 2020 May 26;13(633):eaay8203. doi: 10.1126/scisignal.aay8203. Sci Signal. 2020. PMID: 32457115 Free PMC article.
-
Crosstalk Between Bile Acids and Intestinal Epithelium: Multidimensional Roles of Farnesoid X Receptor and Takeda G Protein Receptor 5.Int J Mol Sci. 2025 Apr 29;26(9):4240. doi: 10.3390/ijms26094240. Int J Mol Sci. 2025. PMID: 40362481 Free PMC article. Review.
References
-
- Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. (2009) Physiol. Rev. 89, 147–191 - PubMed
-
- Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. (1999) Science 284, 1362–1365 - PubMed
-
- Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. (1999) Mol. Cell 3, 543–553 - PubMed
-
- Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., Lehmann J. M. (1999) Science 284, 1365–1368 - PubMed
-
- Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. (2000) Cell 102, 731–744 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

