The loss of Cbl-phosphatidylinositol 3-kinase interaction perturbs RANKL-mediated signaling, inhibiting bone resorption and promoting osteoclast survival
- PMID: 20851882
- PMCID: PMC2978603
- DOI: 10.1074/jbc.M110.124628
The loss of Cbl-phosphatidylinositol 3-kinase interaction perturbs RANKL-mediated signaling, inhibiting bone resorption and promoting osteoclast survival
Abstract
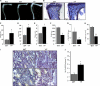
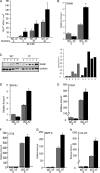
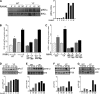
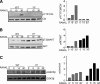
Cbl is an adaptor protein and an E3 ligase that plays both positive and negative roles in several signaling pathways that affect various cellular functions. Tyrosine 737 is unique to Cbl and is phosphorylated by Syk and Src family kinases. Phosphorylated Cbl Tyr(737) creates a binding site for the p85 regulatory subunit of PI3K, which also plays an important role in the regulation of bone resorption by osteoclasts. To investigate the role of Cbl-PI3K interaction in bone homeostasis, we examined the knock-in mice (Cbl(YF/YF)) in which the PI3K binding site in Cbl is ablated due to the mutation in the regulatory tyrosine. We report that in Cbl(YF/YF) mice, despite increased numbers of osteoclasts, bone volume is increased due to defective osteoclast function. Additionally, in ex vivo cultures, mature Cbl(YF/YF) osteoclasts showed an increased ability to survive in the presence of RANKL due to delayed onset of apoptosis. RANKL-mediated signaling is perturbed in Cbl(YF/YF) osteoclasts, and most interestingly, AKT phosphorylation is up-regulated, suggesting that the lack of PI3K sequestration by Cbl results in increased survival and decreased bone resorption. Cumulatively, these in vivo and in vitro results show that, on one hand, binding of Cbl to PI3K negatively regulates osteoclast differentiation, survival, and signaling events (e.g. AKT phosphorylation), whereas on the other hand it positively influences osteoclast function.
Figures

Similar articles
-
Abrogation of Cbl-PI3K interaction increases bone formation and osteoblast proliferation.Calcif Tissue Int. 2011 Nov;89(5):396-410. doi: 10.1007/s00223-011-9531-z. Epub 2011 Sep 28. Calcif Tissue Int. 2011. PMID: 21952831 Free PMC article.
-
Loss of Cbl-PI3K interaction enhances osteoclast survival due to p21-Ras mediated PI3K activation independent of Cbl-b.J Cell Biochem. 2014 Jul;115(7):1277-89. doi: 10.1002/jcb.24779. J Cell Biochem. 2014. PMID: 24470255 Free PMC article.
-
Cbl-PI3K interaction regulates Cathepsin K secretion in osteoclasts.Bone. 2019 Oct;127:376-385. doi: 10.1016/j.bone.2019.07.009. Epub 2019 Jul 9. Bone. 2019. PMID: 31299383 Free PMC article.
-
Cbl-phosphatidylinositol 3 kinase interaction differentially regulates macrophage colony-stimulating factor-mediated osteoclast survival and cytoskeletal reorganization.Ann N Y Acad Sci. 2010 Mar;1192:376-84. doi: 10.1111/j.1749-6632.2009.05346.x. Ann N Y Acad Sci. 2010. PMID: 20392263 Review.
-
The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function.Immunol Rev. 2005 Dec;208:106-25. doi: 10.1111/j.0105-2896.2005.00335.x. Immunol Rev. 2005. PMID: 16313344 Review.
Cited by
-
Protein tyrosine kinase regulation by ubiquitination: critical roles of Cbl-family ubiquitin ligases.Biochim Biophys Acta. 2013 Jan;1833(1):122-39. doi: 10.1016/j.bbamcr.2012.10.010. Epub 2012 Oct 17. Biochim Biophys Acta. 2013. PMID: 23085373 Free PMC article. Review.
-
Loss of protein kinase C-δ protects against LPS-induced osteolysis owing to an intrinsic defect in osteoclastic bone resorption.PLoS One. 2013 Aug 8;8(8):e70815. doi: 10.1371/journal.pone.0070815. eCollection 2013. PLoS One. 2013. PMID: 23951014 Free PMC article.
-
The Hajdu Cheney Mutation Is a Determinant of B-Cell Allocation of the Splenic Marginal Zone.Am J Pathol. 2018 Jan;188(1):149-159. doi: 10.1016/j.ajpath.2017.09.010. Epub 2017 Oct 14. Am J Pathol. 2018. PMID: 29037852 Free PMC article.
-
Abrogation of Cbl-PI3K interaction increases bone formation and osteoblast proliferation.Calcif Tissue Int. 2011 Nov;89(5):396-410. doi: 10.1007/s00223-011-9531-z. Epub 2011 Sep 28. Calcif Tissue Int. 2011. PMID: 21952831 Free PMC article.
-
Signaling pathways associated with Lgr6 to regulate osteogenesis.Bone. 2024 Oct;187:117207. doi: 10.1016/j.bone.2024.117207. Epub 2024 Jul 19. Bone. 2024. PMID: 39033993 Free PMC article.
References
-
- Teitelbaum S. L. (2000) Science 289, 1504–1508 - PubMed
-
- Horne W. C., Sanjay A., Bruzzaniti A., Baron R. (2005) Immunol. Rev. 208, 106–125 - PubMed
-
- Swaminathan G., Tsygankov A. Y. (2006) J. Cell. Physiol. 209, 21–43 - PubMed
-
- Andoniou C. E., Thien C. B., Langdon W. Y. (1996) Oncogene 12, 1981–1989 - PubMed
-
- Marengère L. E., Mirtsos C., Kozieradzki I., Veillette A., Mak T. W., Penninger J. M. (1997) J. Immunol. 159, 70–76 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

