Rapid fusion and syncytium formation of heterologous cells upon expression of the FGFRL1 receptor
- PMID: 20851884
- PMCID: PMC2988375
- DOI: 10.1074/jbc.M110.140517
Rapid fusion and syncytium formation of heterologous cells upon expression of the FGFRL1 receptor
Abstract
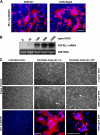
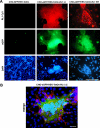
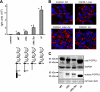
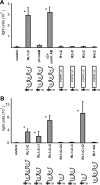
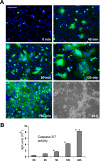
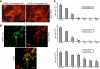
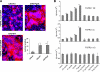
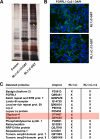
The fusion of mammalian cells into syncytia is a developmental process that is tightly restricted to a limited subset of cells. Besides gamete and placental trophoblast fusion, only macrophages and myogenic stem cells fuse into multinucleated syncytia. In contrast to viral cell fusion, which is mediated by fusogenic glycoproteins that actively merge membranes, mammalian cell fusion is poorly understood at the molecular level. A variety of mammalian transmembrane proteins, among them many of the immunoglobulin superfamily, have been implicated in cell-cell fusion, but none has been shown to actively fuse cells in vitro. Here we report that the FGFRL1 receptor, which is up-regulated during the differentiation of myoblasts into myotubes, fuses cultured cells into large, multinucleated syncytia. We used luciferase and GFP-based reporter assays to confirm cytoplasmic mixing and to identify the fusion inducing domain of FGFRL1. These assays revealed that Ig-like domain III and the transmembrane domain are both necessary and sufficient to rapidly fuse CHO cells into multinucleated syncytia comprising several hundred nuclei. Moreover, FGFRL1 also fused HEK293 and HeLa cells with untransfected CHO cells. Our data show that FGFRL1 is the first mammalian protein that is capable of inducing syncytium formation of heterologous cells in vitro.
Figures
Similar articles
-
Biology of FGFRL1, the fifth fibroblast growth factor receptor.Cell Mol Life Sci. 2011 Mar;68(6):951-64. doi: 10.1007/s00018-010-0576-3. Epub 2010 Nov 16. Cell Mol Life Sci. 2011. PMID: 21080029 Free PMC article. Review.
-
Evolution of the fusogenic activity of the receptor FGFRL1.Arch Biochem Biophys. 2017 Jul 1;625-626:54-64. doi: 10.1016/j.abb.2017.06.002. Epub 2017 Jun 5. Arch Biochem Biophys. 2017. PMID: 28596102
-
Cell-cell fusion induced by the Ig3 domain of receptor FGFRL1 in CHO cells.Biochim Biophys Acta. 2015 Oct;1853(10 Pt A):2273-85. doi: 10.1016/j.bbamcr.2015.05.027. Epub 2015 May 27. Biochim Biophys Acta. 2015. PMID: 26025674
-
A net-like structure with pores is observed during cell fusion induced by the receptor FGFRL1.Commun Integr Biol. 2011 May;4(3):287-90. doi: 10.4161/cib.4.3.14892. Commun Integr Biol. 2011. PMID: 21980560 Free PMC article.
-
Acting on identity: Myoblast fusion and the formation of the syncytial muscle fiber.Semin Cell Dev Biol. 2017 Dec;72:45-55. doi: 10.1016/j.semcdb.2017.10.033. Epub 2017 Nov 6. Semin Cell Dev Biol. 2017. PMID: 29101004 Free PMC article. Review.
Cited by
-
Receptor FGFRL1 does not promote cell proliferation but induces cell adhesion.Int J Mol Med. 2016 Jul;38(1):30-8. doi: 10.3892/ijmm.2016.2601. Epub 2016 May 23. Int J Mol Med. 2016. PMID: 27220341 Free PMC article.
-
Biology of FGFRL1, the fifth fibroblast growth factor receptor.Cell Mol Life Sci. 2011 Mar;68(6):951-64. doi: 10.1007/s00018-010-0576-3. Epub 2010 Nov 16. Cell Mol Life Sci. 2011. PMID: 21080029 Free PMC article. Review.
-
[Expression of fibroblast growth factor receptor like 1 protein in oral squamous cell carcinoma and its influence on tumor cell proliferation and migration].Hua Xi Kou Qiang Yi Xue Za Zhi. 2020 Oct 1;38(5):558-565. doi: 10.7518/hxkq.2020.05.015. Hua Xi Kou Qiang Yi Xue Za Zhi. 2020. PMID: 33085242 Free PMC article. Chinese.
-
Dissecting the Interaction of FGF8 with Receptor FGFRL1.Biomolecules. 2020 Oct 1;10(10):1399. doi: 10.3390/biom10101399. Biomolecules. 2020. PMID: 33019532 Free PMC article.
-
Evidence that the novel receptor FGFRL1 signals indirectly via FGFR1.Int J Mol Med. 2013 Nov;32(5):983-8. doi: 10.3892/ijmm.2013.1484. Epub 2013 Sep 10. Int J Mol Med. 2013. PMID: 24026051 Free PMC article.
References
-
- Chen E. H., Grote E., Mohler W., Vignery A. (2007) FEBS Lett. 581, 2181–2193 - PubMed
-
- Oren-Suissa M., Podbilewicz B. (2007) Trends Cell Biol. 17, 537–546 - PubMed
-
- Martens S., McMahon H. T. (2008) Nat. Rev. Mol. Cell Biol. 9, 543–556 - PubMed
-
- Podbilewicz B., Leikina E., Sapir A., Valansi C., Suissa M., Shemer G., Chernomordik L. V. (2006) Dev. Cell 11, 471–481 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

