The archaeal topoisomerase reverse gyrase is a helix-destabilizing protein that unwinds four-way DNA junctions
- PMID: 20851892
- PMCID: PMC2978581
- DOI: 10.1074/jbc.M110.169029
The archaeal topoisomerase reverse gyrase is a helix-destabilizing protein that unwinds four-way DNA junctions
Abstract
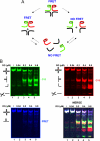
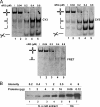
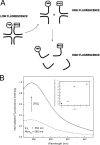
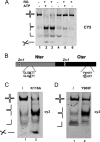
Four-way junctions are non-B DNA structures that originate as intermediates of recombination and repair (Holliday junctions) or from the intrastrand annealing of palindromic sequences (cruciforms). These structures have important functional roles but may also severely interfere with DNA replication and other genetic processes; therefore, they are targeted by regulatory and architectural proteins, and dedicated pathways exist for their removal. Although it is well known that resolution of Holliday junctions occurs either by recombinases or by specialized helicases, less is known on the mechanisms dealing with secondary structures in nucleic acids. Reverse gyrase is a DNA topoisomerase, specific to microorganisms living at high temperatures, which comprises a type IA topoisomerase fused to an SF2 helicase-like module and catalyzes ATP hydrolysis-dependent DNA positive supercoiling. Reverse gyrase is likely involved in regulation of DNA structure and stability and might also participate in the cell response to DNA damage. By applying FRET technology to multiplex fluorophore gel imaging, we show here that reverse gyrase induces unwinding of synthetic four-way junctions as well as forked DNA substrates, following a mechanism independent of both the ATPase and the strand-cutting activity of the enzyme. The reaction requires high temperature and saturating protein concentrations. Our results suggest that reverse gyrase works like an ATP-independent helix-destabilizing protein specific for branched DNA structures. The results are discussed in light of reverse gyrase function and their general relevance for protein-mediated unwinding of complex DNA structures.
Figures
Similar articles
-
The reverse gyrase from Pyrobaculum calidifontis, a novel extremely thermophilic DNA topoisomerase endowed with DNA unwinding and annealing activities.J Biol Chem. 2014 Feb 7;289(6):3231-43. doi: 10.1074/jbc.M113.517649. Epub 2013 Dec 17. J Biol Chem. 2014. PMID: 24347172 Free PMC article.
-
Adenosine 5'-O-(3-thio)triphosphate (ATPgammaS) promotes positive supercoiling of DNA by T. maritima reverse gyrase.J Mol Biol. 2007 Aug 3;371(1):197-209. doi: 10.1016/j.jmb.2007.05.031. Epub 2007 May 18. J Mol Biol. 2007. PMID: 17560602
-
Reverse gyrase: an unusual DNA manipulator of hyperthermophilic organisms.Ital J Biochem. 2007 Jun;56(2):103-9. Ital J Biochem. 2007. PMID: 17722650 Review.
-
Reverse gyrase transiently unwinds double-stranded DNA in an ATP-dependent reaction.J Mol Biol. 2013 Jan 9;425(1):32-40. doi: 10.1016/j.jmb.2012.10.016. Epub 2012 Nov 1. J Mol Biol. 2013. PMID: 23123378
-
Reverse gyrase and genome stability in hyperthermophilic organisms.Biochem Soc Trans. 2009 Feb;37(Pt 1):69-73. doi: 10.1042/BST0370069. Biochem Soc Trans. 2009. PMID: 19143604 Review.
Cited by
-
How Do Thermophiles Organize Their Genomes?Microbes Environ. 2024;39(5):ME23087. doi: 10.1264/jsme2.ME23087. Microbes Environ. 2024. PMID: 38839371 Free PMC article. Review.
-
Synergic and opposing activities of thermophilic RecQ-like helicase and topoisomerase 3 proteins in Holliday junction processing and replication fork stabilization.J Biol Chem. 2012 Aug 31;287(36):30282-95. doi: 10.1074/jbc.M112.366377. Epub 2012 Jun 21. J Biol Chem. 2012. PMID: 22722926 Free PMC article.
-
Insight into the cellular involvement of the two reverse gyrases from the hyperthermophilic archaeon Sulfolobus solfataricus.BMC Mol Biol. 2014 Sep 9;15:18. doi: 10.1186/1471-2199-15-18. BMC Mol Biol. 2014. PMID: 25200003 Free PMC article.
-
In vivo and in vitro protein imaging in thermophilic archaea by exploiting a novel protein tag.PLoS One. 2017 Oct 3;12(10):e0185791. doi: 10.1371/journal.pone.0185791. eCollection 2017. PLoS One. 2017. PMID: 28973046 Free PMC article.
-
Genome stability: recent insights in the topoisomerase reverse gyrase and thermophilic DNA alkyltransferase.Extremophiles. 2014 Sep;18(5):895-904. doi: 10.1007/s00792-014-0662-9. Epub 2014 Aug 8. Extremophiles. 2014. PMID: 25102812 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

