Cadaverine covalently linked to peptidoglycan is required for interaction between the peptidoglycan and the periplasm-exposed S-layer-homologous domain of major outer membrane protein Mep45 in Selenomonas ruminantium
- PMID: 20851903
- PMCID: PMC2976460
- DOI: 10.1128/JB.00417-10
Cadaverine covalently linked to peptidoglycan is required for interaction between the peptidoglycan and the periplasm-exposed S-layer-homologous domain of major outer membrane protein Mep45 in Selenomonas ruminantium
Abstract
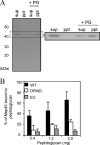
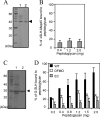
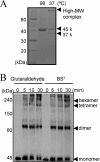
The peptidoglycan of Selenomonas ruminantium is covalently bound to cadaverine (PG-cadaverine), which likely plays a significant role in maintaining the integrity of the cell surface structure. The outer membrane of this bacterium contains a 45-kDa major protein (Mep45) that is a putative peptidoglycan-associated protein. In this report, we determined the nucleotide sequence of the mep45 gene and investigated the relationship between PG-cadaverine, Mep45, and the cell surface structure. Amino acid sequence analysis showed that Mep45 is comprised of an N-terminal S-layer-homologous (SLH) domain followed by α-helical coiled-coil region and a C-terminal β-strand-rich region. The N-terminal SLH domain was found to be protruding into the periplasmic space and was responsible for binding to peptidoglycan. It was determined that Mep45 binds to the peptidoglycan in a manner dependent on the presence of PG-cadaverine. Electron microscopy revealed that defective PG-cadaverine decreased the structural interactions between peptidoglycan and the outer membrane, consistent with the proposed role for PG-cadaverine. The C-terminal β-strand-rich region of Mep45 was predicted to be a membrane-bound unit of the 14-stranded β-barrel structure. Here we propose that PG-cadaverine possesses functional importance to facilitate the structural linkage between peptidoglycan and the outer membrane via specific interaction with the SLH domain of Mep45.
Figures
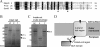


Similar articles
-
Peptidoglycan-associated outer membrane protein Mep45 of rumen anaerobe Selenomonas ruminantium forms a non-specific diffusion pore via its C-terminal transmembrane domain.Biosci Biotechnol Biochem. 2016 Oct;80(10):1954-9. doi: 10.1080/09168451.2016.1194185. Epub 2016 Jun 7. Biosci Biotechnol Biochem. 2016. PMID: 27310312 Free PMC article.
-
Cadaverine covalently linked to the peptidoglycan serves as the correct constituent for the anchoring mechanism between the outer membrane and peptidoglycan in Selenomonas ruminantium.J Bacteriol. 2011 May;193(9):2347-50. doi: 10.1128/JB.00106-11. Epub 2011 Mar 11. J Bacteriol. 2011. PMID: 21398553 Free PMC article.
-
Molecular basis for the maintenance of envelope integrity in Selenomonas ruminantium: cadaverine biosynthesis and covalent modification into the peptidoglycan play a major role.J Nutr Sci Vitaminol (Tokyo). 2012;58(3):153-60. doi: 10.3177/jnsv.58.153. J Nutr Sci Vitaminol (Tokyo). 2012. PMID: 22878384 Review.
-
Molecular dissection of the Selenomonas ruminantium cell envelope and lysine decarboxylase involved in the biosynthesis of a polyamine covalently linked to the cell wall peptidoglycan layer.Biosci Biotechnol Biochem. 2004 Jan;68(1):1-19. doi: 10.1271/bbb.68.1. Biosci Biotechnol Biochem. 2004. PMID: 14745158 Review.
-
Characterization of a major envelope protein from the rumen anaerobe Selenomonas ruminantium OB268.Can J Microbiol. 2000 Apr;46(4):295-303. doi: 10.1139/w99-149. Can J Microbiol. 2000. PMID: 10779865
Cited by
-
Phylogenomic analysis supports the ancestral presence of LPS-outer membranes in the Firmicutes.Elife. 2016 Aug 31;5:e14589. doi: 10.7554/eLife.14589. Elife. 2016. PMID: 27580370 Free PMC article.
-
Peptidoglycan-associated outer membrane protein Mep45 of rumen anaerobe Selenomonas ruminantium forms a non-specific diffusion pore via its C-terminal transmembrane domain.Biosci Biotechnol Biochem. 2016 Oct;80(10):1954-9. doi: 10.1080/09168451.2016.1194185. Epub 2016 Jun 7. Biosci Biotechnol Biochem. 2016. PMID: 27310312 Free PMC article.
-
Outer Membrane Permeability of Cyanobacterium Synechocystis sp. Strain PCC 6803: Studies of Passive Diffusion of Small Organic Nutrients Reveal the Absence of Classical Porins and Intrinsically Low Permeability.J Bacteriol. 2017 Sep 5;199(19):e00371-17. doi: 10.1128/JB.00371-17. Print 2017 Oct 1. J Bacteriol. 2017. PMID: 28696278 Free PMC article.
-
Outer Membrane Proteins Derived from Non-cyanobacterial Lineage Cover the Peptidoglycan of Cyanophora paradoxa Cyanelles and Serve as a Cyanelle Diffusion Channel.J Biol Chem. 2016 Sep 16;291(38):20198-209. doi: 10.1074/jbc.M116.746131. Epub 2016 Aug 8. J Biol Chem. 2016. PMID: 27502278 Free PMC article.
-
A multidomain connector links the outer membrane and cell wall in phylogenetically deep-branching bacteria.Proc Natl Acad Sci U S A. 2022 Aug 16;119(33):e2203156119. doi: 10.1073/pnas.2203156119. Epub 2022 Aug 9. Proc Natl Acad Sci U S A. 2022. PMID: 35943982 Free PMC article.
References
-
- Cava, F., M. A. dePedro, H. Schwarz, A. Henne, and J. Berenguer. 2004. Binding to pyruvylated compounds as an ancestral mechanism to anchor the outer envelope in primitive bacteria. Mol. Microbiol. 52:677-690. - PubMed
-
- Engelhardt, H., and J. Peters. 1998. Structural research on surface layers: a focus on stability, surface layer homology domains, and surface layer-cell wall interactions. J. Struct. Biol. 124:276-302. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

