Seasonal changes of whole root system conductance by a drought-tolerant grape root system
- PMID: 20851906
- PMCID: PMC2993904
- DOI: 10.1093/jxb/erq247
Seasonal changes of whole root system conductance by a drought-tolerant grape root system
Abstract
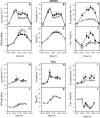
The role of root systems in drought tolerance is a subject of very limited information compared with above-ground responses. Adjustments to the ability of roots to supply water relative to shoot transpiration demand is proposed as a major means for woody perennial plants to tolerate drought, and is often expressed as changes in the ratios of leaf to root area (A(L):A(R)). Seasonal root proliferation in a directed manner could increase the water supply function of roots independent of total root area (A(R)) and represents a mechanism whereby water supply to demand could be increased. To address this issue, seasonal root proliferation, stomatal conductance (g(s)) and whole root system hydraulic conductance (k(r)) were investigated for a drought-tolerant grape root system (Vitis berlandieri×V. rupestris cv. 1103P) and a non-drought-tolerant root system (Vitis riparia×V. rupestris cv. 101-14Mgt), upon which had been grafted the same drought-sensitive clone of Vitis vinifera cv. Merlot. Leaf water potentials (ψ(L)) for Merlot grafted onto the 1103P root system (-0.91±0.02 MPa) were +0.15 MPa higher than Merlot on 101-14Mgt (-1.06±0.03 MPa) during spring, but dropped by approximately -0.4 MPa from spring to autumn, and were significantly lower by -0.15 MPa (-1.43±0.02 MPa) than for Merlot on 101-14Mgt (at -1.28±0.02 MPa). Surprisingly, g(s) of Merlot on the drought-tolerant root system (1103P) was less down-regulated and canopies maintained evaporative fluxes ranging from 35-20 mmol vine(-1) s(-1) during the diurnal peak from spring to autumn, respectively, three times greater than those measured for Merlot on the drought-sensitive rootstock 101-14Mgt. The drought-tolerant root system grew more roots at depth during the warm summer dry period, and the whole root system conductance (k(r)) increased from 0.004 to 0.009 kg MPa(-1) s(-1) during that same time period. The changes in k(r) could not be explained by xylem anatomy or conductivity changes of individual root segments. Thus, the manner in which drought tolerance was conveyed to the drought-sensitive clone appeared to arise from deep root proliferation during the hottest and driest part of the season, rather than through changes in xylem structure, xylem density or stomatal regulation. This information can be useful to growers on a site-specific basis in selecting rootstocks for grape clonal material (scions) grafted to them.
Figures
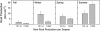
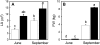


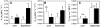
References
-
- Addington RN, Donovan LA, Mitchell RJ, Vose JM, Pecot SD, Jack SB, Hacke UG, Sperry JS, Oren R. Adjustments in hydraulic architecture of Pinus palustris maintain similar stomatal conductance in xeric and mesic habitats. Plant, Cell and Environment. 2006;29:535–545. - PubMed
-
- Alder NN, Sperry JS, Pockmanm WT. Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia. 1996;105:293–301. - PubMed
-
- André J. A study of the vascular organization of bamboos (Poaceae, Bambuseae) using a microcasting method. IAWA Journal. 1998;19:265–278.
-
- Bauerle TL, Eissenstat DM, Granett J, Gardner DM, Smart DR. Consequences of grape phylloxera infestations on root survivorship and root age structure in a California vineyard. Plant, Cell and Environment. 2007;30:786–795. - PubMed
-
- Bauerle TL, Richards JH, Smart DR, Eissenstat DM. Importance of internal hydraulic redistribution for prolonging the lifespan of roots in dry soil. Plant, Cell and Environment. 2008a;31:177–186. - PubMed

