lacZ reporter system for use in Borrelia burgdorferi
- PMID: 20851957
- PMCID: PMC2976203
- DOI: 10.1128/AEM.01389-10
lacZ reporter system for use in Borrelia burgdorferi
Abstract
Regulation of gene expression is critical for the ability of Borrelia burgdorferi to adapt to different environments during its natural infectious cycle. Reporter genes have been used successfully to study gene regulation in multiple organisms. We have introduced a lacZ gene into B. burgdorferi, and we show that B. burgdorferi produces a protein with detectable β-galactosidase activity in both liquid and solid media when lacZ is expressed from a constitutive promoter. Furthermore, when lacZ is expressed from the ospC promoter, β-galactosidase activity is detected only in B. burgdorferi clones that express ospC, and it accurately monitors endogenous gene expression. The addition of lacZ to the repertoire of genetic tools available for use in B. burgdorferi should contribute to a better understanding of how B. burgdorferi gene expression is regulated during the infectious cycle.
Figures
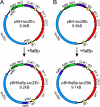

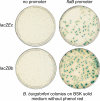
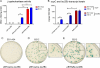
Similar articles
-
A conservative amino acid change alters the function of BosR, the redox regulator of Borrelia burgdorferi.Mol Microbiol. 2004 Dec;54(5):1352-63. doi: 10.1111/j.1365-2958.2004.04352.x. Mol Microbiol. 2004. PMID: 15554974
-
An enhanced GFP reporter system to monitor gene expression in Borrelia burgdorferi.Microbiology (Reading). 2003 Jul;149(Pt 7):1819-1828. doi: 10.1099/mic.0.26165-0. Microbiology (Reading). 2003. PMID: 12855733
-
Analysis of promoter elements involved in the transcriptional initiation of RpoS-dependent Borrelia burgdorferi genes.J Bacteriol. 2004 Nov;186(21):7390-402. doi: 10.1128/JB.186.21.7390-7402.2004. J Bacteriol. 2004. PMID: 15489451 Free PMC article.
-
Transposon mutagenesis as an approach to improved understanding of Borrelia pathogenesis and biology.Front Cell Infect Microbiol. 2014 May 20;4:63. doi: 10.3389/fcimb.2014.00063. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24904839 Free PMC article. Review.
-
Adaptation of Borrelia burgdorferi in the tick and the mammalian host.FEMS Microbiol Rev. 2003 Oct;27(4):493-504. doi: 10.1016/S0168-6445(03)00036-6. FEMS Microbiol Rev. 2003. PMID: 14550942 Review.
Cited by
-
Regulation of the virulence determinant OspC by bbd18 on linear plasmid lp17 of Borrelia burgdorferi.J Bacteriol. 2011 Oct;193(19):5365-73. doi: 10.1128/JB.01496-10. Epub 2011 Jul 22. J Bacteriol. 2011. PMID: 21784941 Free PMC article.
-
Use of an endogenous plasmid locus for stable in trans complementation in Borrelia burgdorferi.Appl Environ Microbiol. 2015 Feb;81(3):1038-46. doi: 10.1128/AEM.03657-14. Epub 2014 Dec 1. Appl Environ Microbiol. 2015. PMID: 25452278 Free PMC article.
-
A Dual Luciferase Reporter System for B. burgdorferi Measures Transcriptional Activity during Tick-Pathogen Interactions.Front Cell Infect Microbiol. 2017 May 31;7:225. doi: 10.3389/fcimb.2017.00225. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28620587 Free PMC article.
-
Strict Conservation yet Non-Essential Nature of Plasmid Gene bba40 in the Lyme Disease Spirochete Borrelia burgdorferi.Microbiol Spectr. 2023 Jun 15;11(3):e0047723. doi: 10.1128/spectrum.00477-23. Epub 2023 Apr 3. Microbiol Spectr. 2023. PMID: 37010416 Free PMC article.
-
Genetic Manipulation of Borrelia Spp.Curr Top Microbiol Immunol. 2018;415:113-140. doi: 10.1007/82_2017_51. Curr Top Microbiol Immunol. 2018. PMID: 28918538 Free PMC article. Review.
References
-
- Alverson, J., S. F. Bundle, C. D. Sohaskey, M. C. Lybecker, and D. S. Samuels. 2003. Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi. Mol. Microbiol. 48:1665-1677. - PubMed
-
- Beaurepaire, C., and G. Chaconas. 2007. Topology-dependent transcription in linear and circular plasmids of the segmented genome of Borrelia burgdorferi. Mol. Microbiol. 63:443-453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

