A multicenter, randomized, open-label, pharmacokinetics and safety study of pantoprazole tablets in children and adolescents aged 6 through 16 years with gastroesophageal reflux disease
- PMID: 20852004
- PMCID: PMC3190583
- DOI: 10.1177/0091270010377501
A multicenter, randomized, open-label, pharmacokinetics and safety study of pantoprazole tablets in children and adolescents aged 6 through 16 years with gastroesophageal reflux disease
Abstract
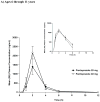
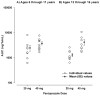
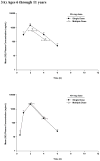
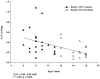
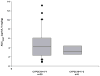
Children with gastroesophageal reflux disease (GERD) may benefit from gastric acid suppression with proton pump inhibitors such as pantoprazole. Effective treatment with pantoprazole requires correct dosing and understanding of the drug's kinetic profile in children. The aim of these studies was to characterize the pharmacokinetic (PK) profile of single and multiple doses of pantoprazole delayed-release tablets in pediatric patients with GERD aged 6 to 11 years (study 1) and 12 to 16 years (study 2). Patients were randomly assigned to receive pantoprazole 20 or 40 mg once daily. Plasma pantoprazole concentrations were obtained at intervals through 12 hours after the single dose and at 2 and 4 hours after multiple doses for PK evaluation. PK parameters were derived by standard noncompartmental methods and examined as a function of both drug dose and patient age. Safety was also monitored. Pantoprazole PK was dose independent (when dose normalized) and similar to PK reported from adult studies. There was no evidence of accumulation with multiple dosing or reports of serious drug-associated adverse events. In children aged 6 to 16 years with GERD, currently available pantoprazole delayed-release tablets can be used to provide systemic exposure similar to that in adults.
Figures


References
-
- Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2009;49:498–547. - PubMed
-
- Sherman PM, Hassall E, Fagundes-Neto U, et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastroenterol. 2009;104:1278–1295. - PubMed
-
- Diaz DM, Winter HS, Colletti RB, et al. Knowledge, attitudes and practice styles of North American pediatricians regarding gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2007;45:56–64. - PubMed
-
- Richter JE, Fraga P, Mack M, Sabesin SM, Bochenek W Pantoprazole US GERD Study Group. Prevention of erosive oesophagitis relapse with pantoprazole. Aliment Pharmacol Ther. 2004;20:567–575. - PubMed
-
- Hassall E, Kerr W, El-Serag HB. Characteristics of children receiving proton pump inhibitors continuously for up to 11 years duration. J Pediatr. 2007;150:262–267.e1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

