Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-I function
- PMID: 20852029
- PMCID: PMC2992784
- DOI: 10.2337/db10-0552
Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-I function
Abstract
Objective: Advanced glycation end products (AGEs) and the renin-angiotensin system (RAS) are both implicated in the development of diabetic retinopathy. How these pathways interact to promote retinal vasculopathy is not fully understood. Glyoxalase-I (GLO-I) is an enzyme critical for the detoxification of AGEs and retinal vascular cell survival. We hypothesized that, in retina, angiotensin II (Ang II) downregulates GLO-I, which leads to an increase in methylglyoxal-AGE formation. The angiotensin type 1 receptor blocker, candesartan, rectifies this imbalance and protects against retinal vasculopathy.
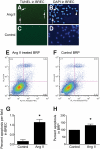
Research design and methods: Cultured bovine retinal endothelial cells (BREC) and bovine retinal pericytes (BRP) were incubated with Ang II (100 nmol/l) or Ang II+candesartan (1 μmol/l). Transgenic Ren-2 rats that overexpress the RAS were randomized to be nondiabetic, diabetic, or diabetic+candesartan (5 mg/kg/day) and studied over 20 weeks. Comparisons were made with diabetic Sprague-Dawley rats.
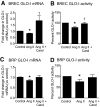
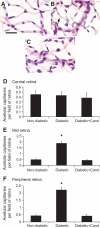
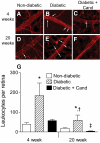
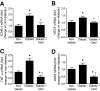
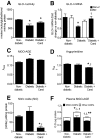
Results: In BREC and BRP, Ang II induced apoptosis and reduced GLO-I activity and mRNA, with a concomitant increase in nitric oxide (NO(•)), the latter being a known negative regulator of GLO-I in BRP. In BREC and BRP, candesartan restored GLO-I and reduced NO(•). Similar events occurred in vivo, with the elevated RAS of the diabetic Ren-2 rat, but not the diabetic Sprague-Dawley rat, reducing retinal GLO-I. In diabetic Ren-2 rats, candesartan reduced retinal acellular capillaries, inflammation, and inducible nitric oxide synthase and NO(•), and restored GLO-I.
Conclusions: We have identified a novel mechanism by which candesartan improves diabetic retinopathy through the restoration of GLO-I.
Figures
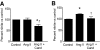
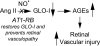
References
-
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
-
- Yamagishi S, Amano S, Inagaki Y, Okamoto T, Koga K, Sasaki N, Yamamoto H, Takeuchi M, Makita Z: Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem Biophys Res Commun 2002;290:973–978 - PubMed
-
- Wilkinson-Berka JL: Angiotensin and diabetic retinopathy. Int J Biochem Cell Biol 2006;38:752–765 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

