Myocardial contraction-relaxation coupling
- PMID: 20852049
- PMCID: PMC3006276
- DOI: 10.1152/ajpheart.00759.2010
Myocardial contraction-relaxation coupling
Abstract
Since the pioneering work of Henry Pickering Bowditch in the late 1800s to early 1900s, cardiac muscle contraction has remained an intensely studied topic for several reasons. The heart is located centrally in our body, and its pumping motion demands the attention of the observer. The contraction of the heart encompasses a complex interplay of mechanical, chemical, and electrical properties, and its function can thus be studied from any of these viewpoints. In addition, diseases of the heart are currently killing more people in the Westernized world than any other disease. When combined with the increasing emphasis of research to be clinically relevant, this contributes to the heart remaining a topic of continued basic and clinical investigation. Yet, there are significant aspects of cardiac muscle contraction that are still not well understood. A big complication of the study of cardiac muscle contraction is that there exists no equilibrium among many of the important governing parameters, which include pre- and afterload, intracellular ion concentrations, membrane potential, and velocity and direction of movement. Thus the classic approach of perturbing an equilibrium or a steady state to learn about the role of the perturbing factor in the system cannot be unambiguously interpreted, since each of the parameters that govern contraction are constantly changing, as well as constantly changing their interaction with each other. In this review, presented as the 54th Bowditch Lecture at Experimental Biology meeting in Anaheim in April 2010, I will revisit several governing factors of cardiac muscle relaxation by applying newly developed tools and protocols to isolated cardiac muscle tissue in which the dynamic interactions between the governing factors of contraction and relaxation can be studied.
Figures
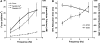
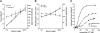
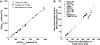
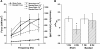
References
-
- Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol 17: 821–840, 1985 - PubMed
-
- Babu GJ, Bhupathy P, Petrashevskaya NN, Wang H, Raman S, Wheeler D, Jagatheesan G, Wieczorek D, Schwartz A, Janssen PM, Ziolo MT, Periasamy M. Targeted overexpression of sarcolipin in the mouse heart decreases sarcoplasmic reticulum calcium transport and cardiac contractility. J Biol Chem 281: 3972–3979, 2006 - PubMed
-
- Bers DM. Ca2+ sparks. Jumping the gap from the cell to cardiac muscle. Circ Res 81: 636–638, 1997 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

