Cell Biology Symposium: imaging the organization and trafficking of lipolytic effectors in adipocytes
- PMID: 20852075
- PMCID: PMC3786141
- DOI: 10.2527/jas.2010-3370
Cell Biology Symposium: imaging the organization and trafficking of lipolytic effectors in adipocytes
Abstract
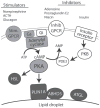
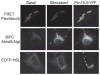
The storage and mobilization of lipid energy are central functions of adipocytes. Lipid energy is stored as triglyceride in lipid droplet structures that are now recognized as bona fide organelles and whose functions are greatly influenced by members of the perilipin family of lipid droplet scaffolds. Recent work indicates that the signaling events underlying fatty acid mobilization involve protein trafficking to a specialized subset of lipid droplets. Furthermore, the core lipolytic machinery is composed of evolutionarily conserved proteins whose functions are conserved in avian and mammalian production species. Lipolysis affects many aspects of animal nutrition and physiology, which can have an important influence on growth efficiency, lactation, and meat quality. This review focuses on recent research that addresses the organization and trafficking of key players in hormone-stimulated lipolysis, and the central role of perilipin1A in adipocyte lipolysis. The review emphasizes recent work from the laboratories of the authors that utilizes imaging techniques to explore the organization and interactions among lipolytic effectors in live cells during lipolytic activation. A mechanistic understanding of lipolysis may lead to new strategies for promoting human and animal health.
Figures

Similar articles
-
Location, location: protein trafficking and lipolysis in adipocytes.Trends Endocrinol Metab. 2008 Jan;19(1):3-9. doi: 10.1016/j.tem.2007.10.006. Epub 2007 Dec 26. Trends Endocrinol Metab. 2008. PMID: 18155916 Review.
-
The central role of perilipin a in lipid metabolism and adipocyte lipolysis.IUBMB Life. 2004 Jul;56(7):379-85. doi: 10.1080/15216540400009968. IUBMB Life. 2004. PMID: 15545214 Review.
-
Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase.J Biol Chem. 2005 Dec 30;280(52):43109-20. doi: 10.1074/jbc.M506336200. Epub 2005 Oct 21. J Biol Chem. 2005. PMID: 16243839
-
Dynamics of lipid droplet-associated proteins during hormonally stimulated lipolysis in engineered adipocytes: stabilization and lipid droplet binding of adipocyte differentiation-related protein/adipophilin.Mol Endocrinol. 2006 Feb;20(2):459-66. doi: 10.1210/me.2005-0323. Epub 2005 Oct 20. Mol Endocrinol. 2006. PMID: 16239256
-
Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis.J Lipid Res. 2007 Dec;48(12):2547-59. doi: 10.1194/jlr.R700014-JLR200. Epub 2007 Sep 18. J Lipid Res. 2007. PMID: 17878492 Review.
Cited by
-
Adipose depots differ in cellularity, adipokines produced, gene expression, and cell systems.Adipocyte. 2014 Dec 10;3(4):236-41. doi: 10.4161/adip.28321. eCollection 2014 Oct-Dec. Adipocyte. 2014. PMID: 26317047 Free PMC article.
-
Vacuolar protein sorting 13C is a novel lipid droplet protein that inhibits lipolysis in brown adipocytes.Mol Metab. 2018 Jan;7:57-70. doi: 10.1016/j.molmet.2017.10.014. Epub 2017 Nov 12. Mol Metab. 2018. PMID: 29175050 Free PMC article.
-
Identification of diverse lipid droplet targeting motifs in the PNPLA family of triglyceride lipases.PLoS One. 2013 May 31;8(5):e64950. doi: 10.1371/journal.pone.0064950. Print 2013. PLoS One. 2013. PMID: 23741432 Free PMC article.
-
Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation.Oncotarget. 2015 Oct 27;6(33):34329-41. doi: 10.18632/oncotarget.6020. Oncotarget. 2015. PMID: 26455377 Free PMC article.
-
Characterization of the bovine gene LIPE and possible influence on fatty acid composition of meat.Meta Gene. 2014 Oct 16;2:746-60. doi: 10.1016/j.mgene.2014.09.001. eCollection 2014 Dec. Meta Gene. 2014. PMID: 25606458 Free PMC article.
References
-
- Akiyama M, Sakai K, Ogawa M, McMillan JR, Sawamura D, Shimizu H. Novel duplication mutation in the patatin domain of adipose triglyceride lipase (PNPLA2) in neutral lipid storage disease with severe myopathy. Muscle Nerve. 2007;36:856–859. - PubMed
-
- Akiyama M, Sawamura D, Nomura Y, Sugawara M, Shimizu H. Truncation of CGI-58 protein causes malformation of lamellar granules resulting in ichthyosis in Dorfman-Chanarin syndrome. J Invest Dermatol. 2003;121:1029–1034. - PubMed
-
- Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. Dynamic activity of lipid droplets: Protein phosphorylation and GTP-mediated protein translocation. J Proteome Res. 2007b;6:3256–3265. - PubMed
-
- Belfrage P, Jergil B, Stralfors P, Tornqvist H. Identification and some characteristics of the enzyme protein of the hormone-sensitive lipase from rat adipose tissue. Adv Exp Med Biol. 1978;101:113–126. - PubMed
-
- Birner-Gruenberger R, Susani-Etzerodt H, Waldhuber M, Riesenhuber G, Schmidinger H, Rechberger G, Kollroser M, Strauss JG, Lass A, Zimmermann R, Haemmerle G, Zechner R, Hermetter A. The lipolytic proteome of mouse adipose tissue. Mol Cell Proteomics. 2005;4:1710–1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

