Disruption of the chaperonin containing TCP-1 function affects protein networks essential for rod outer segment morphogenesis and survival
- PMID: 20852191
- PMCID: PMC3013443
- DOI: 10.1074/mcp.M110.000570
Disruption of the chaperonin containing TCP-1 function affects protein networks essential for rod outer segment morphogenesis and survival
Abstract
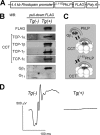
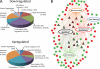
Type II Chaperonin Containing TCP-1 (CCT, also known as TCP-1 Ring Complex, TRiC) is a multi-subunit molecular machine thought to assist in the folding of ∼ 10% of newly translated cytosolic proteins in eukaryotes. A number of proteins folded by CCT have been identified in yeast and cultured mammalian cells, however, the function of this chaperonin in vivo has never been addressed. Here we demonstrate that suppressing the CCT activity in mouse photoreceptors by transgenic expression of a dominant-negative mutant of the CCT cofactor, phosducin-like protein (PhLP), results in the malformation of the outer segment, a cellular compartment responsible for light detection, and triggers rapid retinal degeneration. Investigation of the underlying causes by quantitative proteomics identified distinct protein networks, encompassing ∼ 200 proteins, which were significantly affected by the chaperonin deficiency. Notably among those were several essential proteins crucially engaged in structural support and visual signaling of the outer segment such as peripherin 2, Rom1, rhodopsin, transducin, and PDE6. These data for the first time demonstrate that normal CCT function is ultimately required for the morphogenesis and survival of sensory neurons of the retina, and suggest the chaperonin CCT deficiency as a potential, yet unexplored, cause of neurodegenerative diseases.
Figures


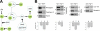
References
-
- Clarke A. R. (2006) Cytosolic chaperonins: a question of promiscuity. Mol. Cell 24, 165–167 - PubMed
-
- Valpuesta J. M., Martín-Benito J., Gómez-Puertas P., Carrascosa J. L., Willison K. R. (2002) Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett. 529, 11–16 - PubMed
-
- Mummert E., Grimm R., Speth V., Eckerskorn C., Schiltz E., Gatenby A. A., Schäfer E. (1993) A TCP1-related molecular chaperone from plants refolds phytochrome to its photoreversible form. Nature 363, 644–648 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

