Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing
- PMID: 20852623
- PMCID: PMC2956429
- DOI: 10.1038/nn.2643
Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing
Abstract
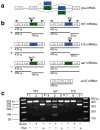
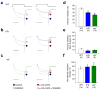
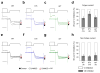
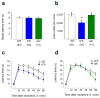
Alternative pre-mRNA splicing occurs extensively in the nervous systems of complex organisms, including humans, considerably expanding the potential size of the proteome. Cell-specific alternative pre-mRNA splicing is thought to optimize protein function for specialized cellular tasks, but direct evidence for this is limited. Transmission of noxious thermal stimuli relies on the activity of N-type Ca(V)2.2 calcium channels in nociceptors. Using an exon-replacement strategy in mice, we show that mutually exclusive splicing patterns in the Ca(V)2.2 gene modulate N-type channel function in nociceptors, leading to a change in morphine analgesia. Exon 37a (e37a) enhances μ-opioid receptor-mediated inhibition of N-type calcium channels by promoting activity-independent inhibition. In the absence of e37a, spinal morphine analgesia is weakened in vivo but the basal response to noxious thermal stimuli is not altered. Our data suggest that highly specialized, discrete cellular responsiveness in vivo can be attributed to alternative splicing events regulated at the level of individual neurons.
Conflict of interest statement
The authors declare no competing financial interests
Figures



Similar articles
-
A functional link between T-type calcium channels and mu-opioid receptor expression in adult primary sensory neurons.J Neurochem. 2009 May;109(3):867-78. doi: 10.1111/j.1471-4159.2009.06014.x. Epub 2009 Feb 24. J Neurochem. 2009. PMID: 19250340 Free PMC article.
-
Spinal morphine but not ziconotide or gabapentin analgesia is affected by alternative splicing of voltage-gated calcium channel CaV2.2 pre-mRNA.Mol Pain. 2013 Dec 26;9:67. doi: 10.1186/1744-8069-9-67. Mol Pain. 2013. PMID: 24369063 Free PMC article.
-
Mu-opioid and GABA(B) receptors modulate different types of Ca2+ currents in rat nodose ganglion neurons.Neuroscience. 1998 Aug;85(3):939-56. doi: 10.1016/s0306-4522(97)00674-x. Neuroscience. 1998. PMID: 9639286
-
Neuronal calcium channels: splicing for optimal performance.Cell Calcium. 2007 Oct-Nov;42(4-5):409-17. doi: 10.1016/j.ceca.2007.04.003. Epub 2007 May 18. Cell Calcium. 2007. PMID: 17512586 Free PMC article. Review.
-
The neuronal splicing factor Nova controls alternative splicing in N-type and P-type CaV2 calcium channels.Channels (Austin). 2010 Nov-Dec;4(6):483-9. doi: 10.4161/chan.4.6.12868. Epub 2010 Nov 1. Channels (Austin). 2010. PMID: 21150296 Free PMC article. Review.
Cited by
-
Cacna1b alternative splicing impacts excitatory neurotransmission and is linked to behavioral responses to aversive stimuli.Mol Brain. 2019 Oct 21;12(1):81. doi: 10.1186/s13041-019-0500-1. Mol Brain. 2019. PMID: 31630675 Free PMC article.
-
Novel coupling is painless.J Gen Physiol. 2014 Apr;143(4):443-7. doi: 10.1085/jgp.201411190. J Gen Physiol. 2014. PMID: 24688017 Free PMC article. No abstract available.
-
Nociceptor Signalling through ion Channel Regulation via GPCRs.Int J Mol Sci. 2019 May 20;20(10):2488. doi: 10.3390/ijms20102488. Int J Mol Sci. 2019. PMID: 31137507 Free PMC article. Review.
-
Heat But Not Mechanical Hypersensitivity Depends on Voltage-Gated CaV2.2 Calcium Channel Activity in Peripheral Axon Terminals Innervating Skin.J Neurosci. 2021 Sep 8;41(36):7546-7560. doi: 10.1523/JNEUROSCI.0195-21.2021. Epub 2021 Aug 5. J Neurosci. 2021. PMID: 34353899 Free PMC article.
-
Interleukin-1α links peripheral CaV2.2 channel activation to rapid adaptive increases in heat sensitivity in skin.bioRxiv [Preprint]. 2024 Mar 31:2023.12.17.572072. doi: 10.1101/2023.12.17.572072. bioRxiv. 2024. Update in: Sci Rep. 2024 Apr 20;14(1):9051. doi: 10.1038/s41598-024-59424-6. PMID: 38585803 Free PMC article. Updated. Preprint.
References
-
- Lipscombe D. Neuronal proteins custom designed by alternative splicing. Curr Opin Neurobiol. 2005;15:358–363. - PubMed
-
- Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nat Rev Neurosci. 2007;8:819–831. - PubMed
-
- Ule J, Darnell RB. RNA binding proteins and the regulation of neuronal synaptic plasticity. Curr Opin Neurobiol. 2006;16:102–110. - PubMed
-
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. - PubMed
-
- Dredge BK, Polydorides AD, Darnell RB. The splice of life: alternative splicing and neurological disease. Nat Rev Neurosci. 2001;2:43–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

